
Micropapillary urothelial carcinoma: is molecular hair-splitting on target?
Urothelial carcinoma (UC) of the bladder has been well investigated in terms of pathogenesis pathways, natural history and tumor biology. Clinically relevant biomarkers including diagnostic, prognostic and predictive molecular markers have been defined in phenotype and genotype analysis beginning with the Cancer Genome atlas (TCGA) study reported in year 2014 (1). The recent 2016 WHO Classification of Tumors of the Urinary System and Male Genital Organs enumerates several histological variants such as the micropapillary, nested, microcystic, plasmacytoid, lymphoepithelioma-like, lipoid cell, clear cell, sarcomatoid and poorly differentiated types (2). Micropapillary urothelial carcinoma (MPUC), first described by Amin et al. in 1994 has generated considerable interest (3). This aggressive variant of UC has a characteristic morphology, aggressive clinical behavior, high propensity for metastasis to regional lymph nodes and distant organs resulting in shorter survival. Recent analysis of molecular, phenotype and microRNA (miRNA) profiles of this variant define unique features which may assist in early recognition and timely treatment (4).
MPUC has a characteristic microscopic morphology with tight small clusters of neoplastic cells in lacunar spaces lacking fibrovascular cores. Nuclei show prominent atypia, large nucleoli, eosinophilic cytoplasm and reverse nuclear polarization in micropapillary clusters with basal secretion of MUC1 (5). Ninety five percent of MPUC tumors have evidence of lymphovascular invasion (6). Heterogeneous morphology and mixed phenotypes exist. Micropapillary genotype and behavior manifests even if a small amount of micropapillary histology (>10%) is present relative to conventional UC (7). Keratin profile in micropapillary carcinoma is similar to conventional UC (Table 1). They are more likely to express cancer antigen 125 (CA 125) indicating glandular differentiation (7). Micropapillary carcinoma also shows positive immunostaining for epithelial membrane antigen, CK7 and CK20, and CD15 (5,17). Metastases are common at the time of initial diagnosis (18). The main differential diagnosis is metastatic serous micropapillary ovarian carcinoma in women or mesothelioma. Fifty six percent of MPUC harbor human epidermal growth factor receptor 2 (HER2) gene amplification which is significantly associated with poor cancer-specific survival rates in patients (14).
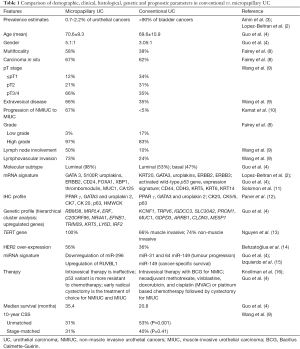
Full table
Several gene profiling studies have reported different sub-categorization of UC. The current accepted grouping reported by Choi et al. 2014, subtypes UC by the use of molecular markers into basal and luminal types in a pattern similar to molecular subtypes of breast carcinoma. The basal subtype is characterized by high expression levels of the markers CD44, KRT5, KRT6B, KRT14. The luminal subtype is enriched for fibroblast growth factor receptor 3 (FGFR3), KRT20, HER2, FOXA1, GATA3, TRIM24, CD24, XBP1, peroxisome proliferator-activated receptor γ (PPAR γ) (19). Alterations of the Rb pathway have been noted mainly in the basal type, while the luminal subtype is characterized by FGFR3 and TSC1 mutations and copy number changes (20). The luminal subtype is associated with better prognosis compared to the basal subtype; however a more aggressive p53-like subset exists within the luminal signature and shows overexpression of p53 (19). MPUC variant shows 98% luminal type molecular profile (4). This variant is consistently positive for expression of markers of terminal luminal differentiation such as GATA3 and uroplakin 2, as well as PPARγ (4).
Preliminary subtypes of UCs were first identified in TCGA study (19) in unsupervised clustering by non-negative matrix factorization of mutations and focal somatic copy number alterations (SCNAs) which identified three groups: group A, highly enriched in focal SCNAs in several genes, as well as mutations in MLL2. Group B, the “papillary CDKN2A-deficient FGFR3 mutant,” enriched in papillary histology with loss of CDKN2A, and 1 or more alterations in FGFR3 and group C, “p53/cell-cycle mutant,” which has p53 mutations in nearly all samples, and enrichment for Rb1 mutations, and amplifications of E2F3 and CCNE1 (20). Clusters I and II both express high HER2 levels and have an elevated estrogen receptor beta signaling signature, suggesting a relationship to HER2-positive breast cancers as well as those of luminal A breast cancer and has high expression of GATA3, FOXA1 and uroplakins. Cluster II differs from cluster I in the absence of papillary morphology or FGFR3 events. In contrast, cluster III (“basal/squamous-like”) is similar in some respects to both basal-like breast cancer which express high levels of keratins 5, 6 and 14 (19,21). These groups are clinically relevant with basal bladder cancers carrying the poorest prognosis and shortest disease-specific survival (19,20). Pathway analysis has led to identification of Stat-3, nuclear factor-κB, HIF-1, and p63 as probable transcriptional drivers of basal gene expression (19) and correspondingly, PPAR-γ and estrogen receptor as drivers of the luminal gene expression pattern.
Low-grade non-muscle invasive urothelial cancers (NMIUC) form approximately 70% of UC. They have a good survival, however they have a tendency to recur and hence require regular monitoring and follow-ups. On the other hand, high-grade muscle-invasive urothelial carcinoma (MIUC) progress rapidly to become metastatic and carry high mortality (19). In terms of invasive and non-invasive UC two divergent pathways of tumorigenesis in bladder cancer are either FGFR3 mutation based or carry p53 mutation. The key genes involved in the FGFR3 pathway are RAS, STAT1, PIK3 and Cyclin D1. These are associated with low grade lesions which carry a low risk of invasion, present at a lower stage have low risk of recurrence and progression and overall carry a good prognosis. The p53 mutation pathway involves Rb gene, p21, bax, bcl2 and TSP1 and is seen in high grade UC and carries a high risk of invasion, tumors present at high stage, recur and progress early in the disease and carry an unfavorable prognosis. NMIUC tumors frequently exhibit FGFR3 and PIK3-kinase catalytic subunit A (PIK3CA) mutations, few chromosomal changes, and low mitotic rate and Ki67 activity. Low-grade non-invasive papillary carcinoma is often multifocal and tends to recur following resection, but rarely progresses to invasive disease. In contrast, micropapillary variant presents with muscle invasive disease in 95% cases (Table 1). Genetic pathways which form targets for therapy are also activated including the mitogen activated protein kinase (MAPK) pathway, phosphoinositide 3-kinase (PI3K) pathway, which affects downstream protein kinase B (AKT) and mammalian target of rapamycin (mTOR) pathways are activated in low grade UC. Upstream of the RAS protein is FGFR3, a tyrosine kinase receptor. FGFR3 or HRAS mutations are present in almost 82% of non-muscle invasive bladder cancer. The more aggressive muscle-invasive tumors carry p53 mutations, and show a high proliferative activity as well as signs of genomic instability. Rb1 deletions and low expression of CDKN2A (p16) forms a parallel pathway in p53 mutated cases. p53 and CDKN1A gene generate p21 protein, a cyclin-dependent kinase inhibitor. A molecular signature combining multiple genes including FGFR3, PIK3CA, KRAS, HRAS, NNRAS, p53, Rb1, CDKN2A and TSC1 correlates well with histologic categories, and can accurately predict whether a tumor fits into noninvasive low-grade papillary or high-grade in situ and invasive groups (22).
The difference and similarities in genotype and phenotype of high grade muscle invasive MPUC and UC have recently been detailed in an interesting study based at the MD Anderson Institute (4). The study compares muscle invasive micropapillary UC, conventional UC and areas of both types of tumors from same patients in two independent publicly available cohorts of conventional UC and micropapillary UC. Two distinct clusters obtained in hierarchical clustering include: cluster A containing UC exclusively and cluster B with mostly micropapillary tumors. MPUC is enriched with expression signatures involved in multiple important oncogenic pathways converging on transformation (mechanisms of cancer, mechanisms of glioma/glioblastoma, RhoA, and p53), cell cycle regulation (cyclins, G1/S checkpoint), DNA damage repair (BRCA1), and signal transduction (ephrin signaling). It is interesting to note that a micropapillary expression signature is also present in the conventional components of the tumors that contained foci of micropapillary carcinoma (4). TERT promoter mutations are present in MPUC, UC with micropapillary areas and conventional UC. Mutations have been identified at positions-124 (C228T) (85%) and -146 (C250T) (12%) upstream of the TERT ATG start site. Concordant mutations have been identified in heterogeneous tumors with MPC and non-MPC areas as well as corresponding conventional UC (13). In view of the similarity in gene signatures within heterogeneous tumors it appears that there is a common oncogenesis origin of UC and its variant histology in individual cases. HER2 protein overexpression or gene amplification has been shown in urothelial bladder cancer. This could be helpful when using targeted anti-HER2 therapy on these tumors. Fifty six percent of MPUC showed HER2 overexpression (3+ staining) while conventional UC show HER2 overexpression in 36% cases and 50% in in-situ carcinoma. All low grade noninvasive tumors have been reported to be HER2 negative (14).
Studies of miRNAs in bladder cancer indicate that their specific species can be associated with bladder cancer behavior and chemosensitivity. Downregulation of miR-296 has been reported in many human cancers. It occurs in later phases of carcinogenesis and is associated with the progression to aggressive disease (23). A conclusive observation in the study by Guo et al. [2016] is the confirmed downregulation of miR-296 in MPUC and the over expression of RUVBL1 (4). MiRNA-296-5p modulation was been shown to be associated with altered viability of cell lines exposed to cisplatin. This explains the chemoresistance encountered in MPUC (24). Similarly, activation of RUVBL1 is associated with clinically aggressive disease (23). The RUVBL1 molecule belongs to the family of AAA+ adenosine triphosphatases which are scaffolding proteins for chromatin-remodeling complexes and control diverse functions including DNA damage repair, proliferation, and invasion (23).
Outcomes of radical cystectomy for patients with MIUC are similar to those with UC when controlling for other clinical and pathologic factors (8). Conventional prognostic parameters include pathologic TNM stage, multifocality and presence of concurrent carcinoma in situ, lymphovascular invasion, histologic grade and adjuvant chemotherapy. Survival analysis using micropapillary gene expression signature with hierarchical clustering shows aggressive behavior is associated with micropapillary tumors as compared to conventional UC (Table 1). The so-called superficial micropapillary carcinoma, which is a high grade MPUC in stage T1, should be offered aggressive therapy instead of intravesical immunotherapy to improve long-term survival (25). The classical morphology and molecular events allow early detection of even a 10% surface micropapillary component and hence MPUC can be detected at an early stage. Prognosis is also related to the proportion and location of the micropapillary component, with higher risk in cases with extensive micropapillary component (7). The p53-like type forms a bad prognosis group with response rates of 45% as compared to 66% in the luminal group, but the difference is statistically insignificant. A small percent of cases with a micropapillary signature exist within a genomically unstable group that overlaps with the luminal and p53-like categories (4). Bacillus Calmette-Guérin (BCG) treatment does not appear to be effective in non-muscle invasive MPUC which progresses in 67% of patients despite intravesical therapy as compared to a progression rate of less than 5% in non-muscle invasive conventional UC (10). Radical cystectomy is hence recommended by some urologic oncologists for even superficial MPUC while others have supported neoadjuvant chemotherapy followed by early cystectomy (6). However concern has been raised related to a potential poor response to cisplatin based neoadjuvant chemotherapy in MPUC (12), a fact explained at MiRNA level by the upregulation of RUVBL1 (4). The so-called p53-ness in MPUC has also been associated with chemoresistance to cisplatin-based neoadjuvant chemotherapy (19).
In view of the key molecular pathways activated in MPUC and UC, potential therapeutic targets and drug interventions include HER2, epidermal growth factor receptor, fibroblast growth factor receptor, programmed cell death ligand 1 (PDL1) and programmed cell death protein 1 (PD1), vascular endothelial growth factor receptor (VEGFR) and vascular endothelial growth factor (VEGF). The receptor tyrosine kinase (RTK)/RAS pathway involved in cell cycle signalling is altered in 44% of tumours and tyrosine kinase inhibitors may form a treatment modality (1,22). Genes involved in regulating chromatin, the structure of DNA and proteins that makes up chromosomes, are frequently mutated and represent novel targets for bladder cancer (1,22). It seems hair splitting of UC into variants with specific molecular signatures will help define targets for therapy. It is an exciting time of translation from bench to bedside in cancer therapeutics and molecular pathologists have the potential to be the guiding hand in determining optimal treatment regimen for patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peng Zhang (Department of Urology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315-22. [Crossref] [PubMed]
- Lopez-Beltran A, Sauter G, Gasser T, et al. Infiltrating urothelial carcinoma. In: Moch H, Humphrey PA, Ulbright TM, et al. editors. WHO Classification of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press, 2016;81-98.
- Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol 1994;18:1224-32. [Crossref] [PubMed]
- Guo CC, Dadhania V, Zhang L, et al. Gene Expression Profile of the Clinically Aggressive Micropapillary Variant of Bladder Cancer. Eur Urol 2016;70:611-620. [Crossref] [PubMed]
- Sangoi AR, Higgins JP, Rouse RV, et al. Immunohistochemical comparison of MUC1, CA125, and Her2Neu in invasive micropapillary carcinoma of the urinary tract and typical invasive urothelial carcinoma with retraction artifact. Mod Pathol 2009;22:660-7. [Crossref] [PubMed]
- McQuitty E, Ro JY, Truong LD, et al. Lymphovascular invasion in micropapillary urothelial carcinoma: a study of 22 cases. Arch Pathol Lab Med 2012;136:635-9. [Crossref] [PubMed]
- Samaratunga H, Khoo K. Micropapillary variant of urothelial carcinoma of the urinary bladder; a clinicopathological and immunohistochemical study. Histopathology 2004;45:55-64. [Crossref] [PubMed]
- Fairey AS, Daneshmand S, Wang L, et al. Impact of micropapillary urothelial carcinoma variant histology on survival after radical cystectomy. Urol Oncol 2014;32:110-6. [Crossref] [PubMed]
- Wang JK, Boorjian SA, Cheville JC, et al. Outcomes following radical cystectomy for micropapillary bladder cancer versus pure urothelial carcinoma: a matched cohort analysis. World J Urol 2012;30:801-6. [Crossref] [PubMed]
- Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer 2007;110:62-7. [Crossref] [PubMed]
- Solomon JP, Lowenthal BM, Kader AK, et al. Challenges in the Diagnosis of Urothelial Carcinoma Variants: Can Emerging Molecular Data Complement Pathology Review? Urology 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Paner GP, Annaiah C, Gulmann C, et al. Immunohistochemical evaluation of novel and traditional markers associated with urothelial differentiation in a spectrum of variants of urothelial carcinoma of the urinary bladder. Hum Pathol 2014;45:1473-82. [Crossref] [PubMed]
- Nguyen D, Taheri D, Springer S, et al. High prevalence of TERT promoter mutations in micropapillary urothelial carcinoma. Virchows Arch 2016;469:427-34. [Crossref] [PubMed]
- Behzatoğlua K, Yörükoğlub K, Demirc H, et al. Human Epidermal Growth Factor Receptor 2 Overexpression in Micropapillary and Other Variants of Urothelial Carcinoma. Eur Urol Focus 2016; Epub ahead of print. [Crossref]
- Izquierdo L, Ingelmo-Torres M, Mallofré C, et al. Prognostic value of microRNA expression pattern in upper tract urothelial carcinoma. BJU Int 2014;113:813-21. [Crossref] [PubMed]
- Knollman H, Godwin JL, Jain R, et al. Muscle-invasive urothelial bladder cancer: an update on systemic therapy. Ther Adv Urol 2015;7:312-30. [Crossref] [PubMed]
- Lopez-Beltran A, Montironi R, Blanca A, et al. Invasive micropapillary urothelial carcinoma of the bladder. Hum Pathol 2010;41:1159-64. [Crossref] [PubMed]
- Hong SP, Park SW, Lee SJ, et al. Bile duct wall metastasis from micropapillary variant transitional cell carcinoma of the urinary bladder mimicking primary hilar cholangiocarcinoma. Gastrointest Endosc 2002;56:756-60. [Crossref] [PubMed]
- Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152-65. [Crossref] [PubMed]
- Martin-Doyle W, Kwiatkowski DJ. Molecular biology of bladder cancer. Hematol Oncol Clin North Am 2015;29:191-203. vii. [Crossref] [PubMed]
- Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014;111:3110-5. [Crossref] [PubMed]
- Solomon JP, Hansel DE. The Emerging Molecular Landscape of Urothelial Carcinoma. Surg Pathol Clin 2016;9:391-404. [Crossref] [PubMed]
- Taniuchi K, Furihata M, Iwasaki S, et al. RUVBL1 directly binds actin filaments and induces formation of cell protrusions to promote pancreatic cancer cell invasion. Int J Oncol 2014;44:1945-54. [PubMed]
- Nordentoft I, Birkenkamp-Demtroder K, Agerbæk M, et al. miRNAs associated with chemo-sensitivity in cell lines and in advanced bladder cancer. BMC Med Genomics 2012;5:40. [Crossref] [PubMed]
- Willis DL, Flaig TW, Hansel DE, et al. Micropapillary bladder cancer: current treatment patterns and review of the literature. Urol Oncol 2014;32:826-32. [Crossref] [PubMed]


