
HIF-pathway proteins: central regulators of tumor immunology
Hypoxia pathway proteins
The master regulators of the cellular response to hypoxia comprise a heterodimeric complex formed by a constitutive nuclear HIFβ, and a cytoplasmic oxygen-dependent HIFα subunit (mainly HIF-1α and HIF-2α). Stabilization of HIFα is regulated by a group of oxygen and iron dependent enzymes, known as HIF-prolyl hydroxylase domain enzymes (PHD1-3). Hence, in normoxia PHDs hydroxylate two prolyl residues on HIFα, which allows binding of the von Hippel-Lindau (VHL) tumor-suppressor protein, leading to subsequent ubiquitination and proteasomal degradation of this subunit [reviewed by (1)]. Consequently, during a period of oxygen deprivation the HIF complex is able to bind specific hypoxia responsive elements (HRE) triggering transcription of a selection of genes implicated in different processes involved in overcoming this hypoxic stress, including erythropoiesis, angiogenesis, metabolism, and inflammation [reviewed by (2-4)] (Figure 1). Interestingly, HIF-1α is ubiquitously expressed whereas HIF-2α is expressed in a more limited fashion; primarily in adult lungs, heart, kidney and liver, and specialized cells like endothelium and erythropoietin producing cells in the kidney (5,6). Regarding the immune system, HIF-1α is expressed in virtually all innate and adaptive immune populations, whereas HIF-2α stabilizes in some immune cells, including monocytes/macrophages. In general, balanced expression of both isoforms has been shown to have a great impact on polarization of T cells and macrophages.
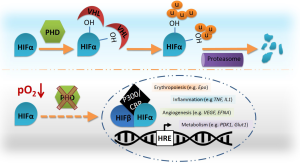
PHD isoforms also present overlapping as well as unique roles. Indeed, PHD2 has been described as the main regulator of the hypoxia pathway, since full PHD2 knock-out embryo’s die around E13.5, whereas PHD1−/− or PHD3−/− mice show a normal development (7) [for a full review on PHD2 see (1)]. On the other hand, several reports demonstrated a certain degree of redundancy depending on the setting and cell type studied. Recent work from our group revealed that conditional loss of PHD2, resulting in a severe form of erythrocytosis is not lethal due to the protective effect of HIF1α-induced overexpression of PHD3 (8). Wu and colleagues reported a similar compensatory function between PHD2 and PHD3 in osteoblast progenitors during a model of bone metabolism in mice (9). Furthermore, the group of Dr. Kaelin showed that only deficiency of all the PHDs in liver cells can lead to increased erythropoietin levels and hematocrit (10). Such a redundant role for all three PHD isoforms has recently been also demonstrated in T lymphocytes. Interestingly, under steady-state conditions only the triple PHD deficient mice (tKO) displayed lung hemorrhage caused by a reduction of induced regulatory T-cells (iTreg) and enhanced CD8+ T cell effector function resulting in diminished tolerance in the lung (11). Although not that many conditional double and triple PHD deficient mouse lines have been described so far, the currently available data suggest a selective redundancy based on functionality and/or cell specificity.
One thing the various studies in this particular field have shown is the complexity of the regulatory network between the different HIFα and PHD isoforms, their downstream effector genes, as well as other pathways that can be involved (e.g., NF-κB, TGFβ) [reviewed by (12)]. Not only does this make it increasingly difficult to determine a general protective and/or detrimental role for these proteins, it also limits the use of appropriate treatments.
Immunity and cancer
The effect of hypoxia on tumor biology has been widely described, since it is an important feature in virtually all solid tumors. Adaptive responses to low oxygen levels by tumor cells are the result of specific genetic programs that strongly regulate survival and proliferation of cancer cells, as well as inducing angiogenesis, immune-surveillance escape, epithelial-to-mesenchymal transition and metastasis. The HIF transcription factors have been described to be of great importance in regulating this tumoral expression profile [reviewed by (13)]. However, this relatively simple model has been challenged in light of data from various studies that reveal unique and sometimes opposing activities of the HIF-1α and HIF-2α isoforms. It is therefore not surprising that also for the PHD isoforms no clear overall mode of action can be deduced. Indeed, an extensive collection of human cancer microarray data (Oncomine database; ThermoFisher Scientific, Waltham, MA, USA) shows overall considerably more cases of PHD2 or PHD3 overexpression, especially in tumors of the kidney, lung and liver. Interestingly, however, cancers related to the intestinal tract and especially colorectal cancer (CRC) show a clear downregulation of these two oxygen sensors, suggesting for a local protective role of these proteins [Table 2 in review by Meneses and Wielockx (1)].
HIF-pathway proteins do not only regulate tumor development from within the cancer cells, they also have a great impact on the tumor microenvironment (TME), including tumor-associated immune cells [reviewed by (14)]. Therefore, many research has been focused on the role the hypoxia pathway plays in two major but seemingly counteracting forces during tumor development: (I) Host immune-related anti-tumor defense, and (II) immune suppression. Regarding the latter, tumor-associated macrophages (TAMs), also known as polarized pro-tumoral M2 cells, constitute the major force helping the tumor to grow. Initial studies in this field were focused on the role of the HIF transcription factors, showing that loss of HIF-1α in TAMs enhances polarization towards an M2 phenotype, attenuating their pro-angiogenic responses. Moreover, these M2-like macrophages overexpress HIF-2α, which has been correlated with poor patient prognosis (15). In line with this study, loss of HIF-2α in macrophages has been reported to decrease TAM infiltration in a murine model of hepatocellular carcinoma (16). In a transgenic breast carcinoma mouse model (MMTV-PyMT), Doedens et al. revealed that the targeted deletion of HIF-1α in macrophages resulted in reduced breast tumor growth through T-cell suppression (17).
Under normal physiological conditions, Treg cells have a critical role in maintaining the homeostasis of innate cytotoxic lymphocytes and regulate the expansion and activation of T and B cells. In cancer, Tregs are another TME cell type that displays diverse immune modulatory functions. Given their complex regulatory roles in response to different environmental stimuli, it is not surprising that Treg cells have diverse effects on tumorigenesis. Indeed, in breast cancer and hepatocellular carcinoma, increased numbers of Treg cells correlate with reduced overall survival, whereas in colorectal cancer Treg cells are associated with improved survival (18). T cell-mediated inflammation and differentiation studies have reported a role for HIF pathway proteins in regulating the balance between Treg and Th17 cells. In this regard, work performed by Dang and colleagues showed that HIF-1α targets FoxP3 for proteasomal degradation, whilst enhancing RORγt, the master-transcription factor driving Th17 differentiation (19). Conversely, HIF-1α is required for an optimal Treg function in order to control T cell-mediated colitis (20). In the recent publication from Clever and colleagues, loss of all three PHDs in T cells led to more active (IFNγ+) CD4+ and CD8+ cells in the lungs of these mice. In addition, the proportion of Treg cells in their hemorrhagic lungs was comparable to wild-type mice, but induced Tregs (iTregs) (Neuropilin-1lo) were lower (11). Although not investigated in the presented work, these tKO cells may reveal, next to a constitutive HIF activity, excessive NFκB signaling, which is known to prevent iTreg cell differentiation (21).
Although several studies have focused on the role of the HIF-pathway proteins and T cell mediated immunity, there is only limited amount of data available in tumor models. Our group reported that loss of PHD2 in myeloid cells and T cells is necessary and sufficient to delay LLC as well as B16 tumor cell growth in mice due to increased cell death of the cancer cells (22). In tKO mice, no difference was detected in the size of the primary B16 tumors. However, these mice were significantly protected from tumor colonization in the lung, which correlated with increased numbers of antitumoral INFγ+ CD4+ T cells. Interestingly, all three oxygen sensors seem to have a negative influence on INFγ expression and its antitumor activity, since neutralization of this induced cytokine in tKO lungs reversed the decreased lung colonization (11). Despite these interesting findings, the study also presents a severe limitation, as these tKO mice already display severe hemorrhage under steady-state conditions. This could potentially influence homing of the injected tumor cells and lead to an altered colonization.
Hypoxia within the neoplastic area also triggers angiogenesis. However, it has been described that new vessels formed in tumors are often disorganized, immature and leaky, which could result in more disseminated tumor cells. Mazzone and colleagues were the first to show that mice heterozygous deficient for PHD2 in endothelial cells are protected from metastasis due to a process known as endothelial normalization (23). Conversely, Branco-Price et al. found that loss of HIF-1α in endothelial cells reduces NO synthesis, retards tumor cell migration through endothelial layers, and restricts tumor cell metastasis. Loss of HIF-2α showed a complete opposite effect (24). Taken together, these studies form another clear example of how targeting the hypoxia pathway can apparently have different effects depending on the targeted cell type and protein.
Translational perspectives of targeting HIF pathway proteins
Over the past years, several studies have reported the use of hypoxia pathway inhibitors for the treatment of inflammatory disorders, including cancer. The different classes of hypoxia pathway inhibitors available to date have been thoroughly described elsewhere (25-27). As mentioned above, the effect of targeting HIF pathway proteins varies depending on the targeted cell type, the condition and even the setting it is used in. This is not surprising, taken into account the variety of different target genes reported for HIF-1α and/or HIF-2α (28), and the distinct pathways these transcription factors can regulate. In this regard, it was shown that specific inhibition of HIF-1α reduced the allergic inflammation and lung remodeling in mice (29), whereas loss of HIF-1α in intestinal epithelial cells increases the severity of clinical symptoms, including vessel permeability. Conversely, constitutive HIF-1α expression due to lack of pHVL in the same cells is protective (30), which was confirmed with specific PHD inhibitors (FG-4497 and FG-4442) (31). A consecutive experiment with DMOG (another PHD inhibitor) led to a decrease in epithelial apoptosis with increased expression of survival protein cIAP through activation of the HIF-1α and NFκB pathway (32). A few years later, it was demonstrated that PHD1 is the detrimental factor in the sensitivity to colitis (33).
In contrast, PHDs seem to have a protective role in CRCs (Oncomine database), which seem to directly relate to a detrimental role for HIF-2α in this tumor type. In this regard, Xue and colleagues showed that loss of VHL induces increased cell survival and proliferation of these tumor cells in a HIF-2α dependent manner (34). Additionally, patients with early-stage CRC and low PHD2 expression displayed poorer survival; an effect that was independent of HIF1α (35). Furthermore, Chan et al. showed that PHD2 silencing in CRC xenografts resulted in an increase of tumor vasculature and primary growth. In this setting, the phenotype was HIF-independent but directly related to NF-κB signaling (36).
Based on these publications and numerous other studies, targeting of the HIF-pathway proteins offers itself as an attractive option to treat inflammation and cancer. Indeed, a number of studies have resulted in clinical trials using various compounds regulating the functionality of these proteins (25-27). There are however several restrictions to overcome during development of efficient cancer therapies: (I) the intricate interactions between HIF and PHD and eventually also compensatory effects; (II) the overlapping and exclusive functions of all the individual proteins; (III) the high heterogeneity of tumor cells; and (IV) limiting side effects to other organs. Therefore, it could be more beneficial to target immune cells in order to enhance anti-tumoral host activity, or improve drug delivery to the neoplastic area. Regarding the latter, vessel normalization might serve as a suitable approach. A recent study showed that PHD2 haplo-deficiency protects organ toxicity in kidney and heart due to oxidative stress upon cisplatin and doxorubicin treatment in a HIF-1α and HIF-2α dependent manner (37). On the other hand, enhancing the anti-tumoral response of the host immune cells seems another appealing approach for cancer treatment, especially with regard to the findings from Clever and colleagues (11). This group showed that ex vivo pre-treatment of T cells with the PHD inhibitor DMOG led to a significant decrease of metastatic nodules in the lung, smaller primary tumors as well as prolonged overall survival. These findings therefore provide a potential new approach to enforce Th1 cell differentiation and improve adoptive cell transfer immunotherapy (ACT) in the treatment of cancer patients. Nevertheless, more research will be necessary to better understand the negative side effects of this ACT on the non-cancerous cells of the lung and its integrity.
Taken together, the central involvement of HIF-pathway proteins during different stages of tumor development and dissemination makes this pathway an attractive target for cancer therapy. Nonetheless, as these proteins have many different and even opposing effects depending on the target and circumstances, more detailed research will be of utmost importance.
Acknowledgments
We would like to thank Ana M. Meneses for critically reading this work.
Funding: Research in the lab of Ben Wielockx has been supported by grants from the DFG (WI 3291/1-1, 1-2, 3 and 5). S.S-G. is funded by a grant from the Dresden International Graduate School for Biomedicine and Bioengineering (DIGS-BB, TU-Dresden).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hao Feng (Experimental Surgical Research, Department of General, Visceral, Transplant, Vascular and Thoracic Surgery, Hospital of the LMU Munich, Germany).
Conflicts of Interest: B.W. is a Heisenberg Professor (DFG). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meneses AM, Wielockx B. PHD2: from hypoxia regulation to disease progression. Hypoxia (Auckl) 2016;4:53-67. [PubMed]
- Mamlouk S, Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer 2013;132:2721-9. [Crossref] [PubMed]
- Franke K, Gassmann M, Wielockx B. Erythrocytosis: the HIF pathway in control. Blood 2013;122:1122-8. [Crossref] [PubMed]
- Singh RP, Franke K, Wielockx B. Hypoxia-mediated regulation of stem cell fate. High Alt Med Biol 2012;13:162-8. [Crossref] [PubMed]
- Rankin EB, Biju MP, Liu Q, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 2007;117:1068-77. [Crossref] [PubMed]
- Wiesener MS, Jürgensen JS, Rosenberger C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J 2003;17:271-3. [PubMed]
- Takeda K, Aguila HL, Parikh NS, et al. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood 2008;111:3229-35. [Crossref] [PubMed]
- Franke K, Kalucka J, Mamlouk S, et al. HIF-1α is a protective factor in conditional PHD2-deficient mice suffering from severe HIF-2α-induced excessive erythropoiesis. Blood 2013;121:1436-45. [Crossref] [PubMed]
- Wu C, Rankin EB, Castellini L, et al. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev 2015;29:817-31. [Crossref] [PubMed]
- Minamishima YA, Kaelin WG Jr. Reactivation of hepatic EPO synthesis in mice after PHD loss. Science 2010;329:407. [Crossref] [PubMed]
- Clever D, Roychoudhuri R, Constantinides MG, et al. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell 2016;166:1117-1131.e14. [Crossref] [PubMed]
- Wong BW, Kuchnio A, Bruning U, et al. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem Sci 2013;38:3-11. [Crossref] [PubMed]
- Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 2011;12:9-22. [PubMed]
- LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol 2016;18:356-65. [Crossref] [PubMed]
- Werno C, Menrad H, Weigert A, et al. Knockout of HIF-1α in tumor-associated macrophages enhances M2 polarization and attenuates their pro-angiogenic responses. Carcinogenesis 2010;31:1863-72. [Crossref] [PubMed]
- Imtiyaz HZ, Williams EP, Hickey MM, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 2010;120:2699-714. [Crossref] [PubMed]
- Doedens AL, Stockmann C, Rubinstein MP, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 2010;70:7465-75. [Crossref] [PubMed]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423-37. [Crossref] [PubMed]
- Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell 2011;146:772-84. [Crossref] [PubMed]
- Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A 2012;109:E2784-93. [Crossref] [PubMed]
- Molinero LL, Miller ML, Evaristo C, et al. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB-dependent manner. J Immunol 2011;186:4609-17. [Crossref] [PubMed]
- Mamlouk S, Kalucka J, Singh RP, et al. Loss of prolyl hydroxylase-2 in myeloid cells and T-lymphocytes impairs tumor development. Int J Cancer 2014;134:849-58. [Crossref] [PubMed]
- Mazzone M, Dettori D, Leite de Oliveira R, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009;136:839-51. [Crossref] [PubMed]
- Branco-Price C, Zhang N, Schnelle M, et al. Endothelial cell HIF-1α and HIF-2α differentially regulate metastatic success. Cancer Cell 2012;21:52-65. [Crossref] [PubMed]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med 2011;365:537-47. [Crossref] [PubMed]
- Wigerup C, Påhlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther 2016;164:152-69. [Crossref] [PubMed]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393-410. [Crossref] [PubMed]
- Schödel J, Oikonomopoulos S, Ragoussis J, et al. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 2011;117:e207-17. [Crossref] [PubMed]
- Huerta-Yepez S, Baay-Guzman GJ, Bebenek IG, et al. Hypoxia inducible factor promotes murine allergic airway inflammation and is increased in asthma and rhinitis. Allergy 2011;66:909-18. [Crossref] [PubMed]
- Karhausen J, Furuta GT, Tomaszewski JE, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 2004;114:1098-106. [Crossref] [PubMed]
- Robinson A, Keely S, Karhausen J, et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 2008;134:145-55. [Crossref] [PubMed]
- Cummins EP, Seeballuck F, Keely SJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 2008;134:156-65. [Crossref] [PubMed]
- Tambuwala MM, Cummins EP, Lenihan CR, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 2010;139:2093-101. [Crossref] [PubMed]
- Xue X, Taylor M, Anderson E, et al. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res 2012;72:2285-93. [Crossref] [PubMed]
- Xie G, Zheng L, Ou J, et al. Low expression of prolyl hydroxylase 2 is associated with tumor grade and poor prognosis in patients with colorectal cancer. Exp Biol Med (Maywood) 2012;237:860-6. [Crossref] [PubMed]
- Chan DA, Kawahara TL, Sutphin PD, et al. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell 2009;15:527-38. [Crossref] [PubMed]
- Leite de Oliveira R, Deschoemaeker S, Henze AT, et al. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell 2012;22:263-77. [Crossref] [PubMed]


