
The role of HER2 overexpression in Middle Eastern papillary thyroid cancer
Introduction
Thyroid cancer is the second most common malignancy among females in Saudi Arabia accounting for 7.4% of all cancers and 10.6% of all female malignant cancers (1) . This is a comparatively higher frequency than in Western countries. For example, thyroid cancer accounts for only 3% of all cancers in the United States (2). Papillary thyroid carcinoma (PTC) is the most common thyroid cancer subtype representing 80–90% of all thyroid malignancies (3).
PTC has an excellent prognosis and is usually cured by current therapy regimens consisting of surgery followed by radio-iodine therapy. However, there is a minor fraction of patients with a dismal course of disease. Tumor recurrence occurs in approximately 5% of PTC and the mortality rate is 1–2% (4,5). Aggressive malignant behavior is strongly related to various clinicopathological variables, including tall cell variant, advanced stage, vascular invasion and nodal or distant metastasis. These parameters are statistically powerful but still not sufficient to predict unfavorable disease course in all patients. A certain prediction of aggressive tumor behavior could potentially be exploited to complement radio-iodine therapy by other cytotoxic treatments.
As our knowledge on the molecular mechanisms involved in thyroid cancer development and progression continuously increase, it can be hoped, that molecular information will eventually contribute to a better initial assessment of tumor’s aggressiveness. Human epidermal growth factor receptor 2 (HER2) is of particular interest in tumor biology as it represents a strong prognostic feature in several tumor types and simultaneously serves as a highly utile therapeutic target in breast and stomach cancer (6,7) along with possibly other cancers (8,9). HER2 encodes a 185-kDa transmembrane kinase glycoprotein (10). HER2 was first reported as overexpressed in human breast and ovarian cancers (11). HER2 overexpression has later been observed in a large variety of other human malignancies originating in different organs, such as stomach, colon, lung and pancreas (12-15).
Data on the role of HER2 in papillary thyroid cancer is not conclusive. Reported incidences of HER2 overexpression vary, from 0 to 79.5%, in studies involving thyroid patients (16-22). Given the generally excellent prognosis of papillary thyroid cancers, and the need for very large patient cohorts to find associations with rare clinical events, robust data on the prognostic role of HER2 expression in papillary thyroid cancer is lacking. Due to the high incidence of papillary thyroid cancer in Saudi Arabia, we were able to collect a cohort of 1,040 papillary thyroid cancer with follow up information. In this project we utilized this patient collection to investigate prevalence and clinical significance of HER2 overexpression in papillary thyroid cancer.
Methods
Patient selection and tissue microarray (TMA) construction
One thousand and forty patients with PTC, diagnosed between 1988 and 2011, were selected from files of the King Faisal Specialist Hospital and Research Centre. All PTC were analyzed in a TMA format. Clinical and histopathological data were available for all the patients. TMAs were constructed with 2-fold redundancy from formalin-fixed, paraffin-embedded PTC specimens as described previously (23). Tumor regions were mapped by a pathologist for coring. The TMA was constructed with 0.6 mm diameter cores spaced 0.8 mm apart using a tissue microarrayer (Semi-automated Arrayer, CM1 Mirlacher, Neuenburg, Germany). The TMA block was cut into 5 µm sections, adhered to a slide by an adhesive tape-transfer method (Instrumedics, Hackensack, NJ) and UV crosslinked. The Institutional Review Board of the King Faisal Specialist Hospital and Research Centre approved the study under Project RAC# 2080-031 on PTC archival clinical samples.
Immunohistochemistry
Standard protocol was followed for IHC staining. For antigen retrieval, Dako Target Retrieval Solution pH 9.0 (Catalog number S2368) was used, and the slides were microwaved at 750 W for 5 min and then at 250 W for 20 min. TMA sections were stained using FDA approved HercepTest kit from DAKO using manufacturers instruction. All slides were counterstained with hematoxylin, dehydrated, cleared and mounted. Negative controls included omission of the primary antibody. Normal tissues of different organ system were also included in the TMA to serve as control. Only fresh cut slides were stained simultaneously to minimize the influence of slide aging and maximize reproducibility of the experiment. For HER2 immunoscoring, DAKO scoring guidelines for gastric cancer were followed as no standard guidelines for HER2 scoring in thyroid tumors exist. Briefly, tumors were categorized into four groups based on intensity score (0, 1+, 2+, 3+). Intensity score 2+ and 3+ was taken as positive, as described previously (19). IHC scoring was done by two pathologists (SB & SP), blinded to the clinicopathological characteristics. Discordant scores were reviewed together to achieve agreement.
Fluorescence in situ hybridization (FISH)
For HER2 dual-color FISH on paraffin-embedded TMA was performed using commercially available DNA probes LSI HER2/CEP 17; Vysis Inc. BX51 Olympus fluorescence microscope (Olympus, Richardson, TX, USA) was used for screening the FISH slides. The HER2 locus specific probe located on chromosome 17, was labeled with Spectrum Orange whilst the centromere was labelled with Spectrum Green (LSI HER2/CEP 17; Vysis Inc.). Histologic TMA tissue sections, 5 µm thick, were deparaffinized with a series of xylene prior to immersion in 100% ethanol. FISH was carried according to the manufacturer’s instructions. FISH scoring was performed independent of the IHC result. The number of HER2 (red) and CEP17 (green) signals were scored for each sample in 20 nuclei. The HER2/CEP17 ratio was calculated according to ASCO/CAP guidelines. A HER2/CEP17 ratio of 1 was considered normal, less than 1.8 as non-amplified and more than 2.2 as amplified (24).
Data analysis and statistics
The JMP 10.0 (SAS Institute Inc., Cary, NC, USA) software package was used for data analyses. We examined the association of HER2 expression with clinicopathological parameters, biomarker expression using chi-square tests and also performed survival analysis by using the Mantel-Cox log-rank test. Survival curves were generated using Kaplan-Meier method with significance evaluated using the Mantel-Cox log-rank test. Values of P<0.05 were considered statistically significant.
Results
Clinicopathological features
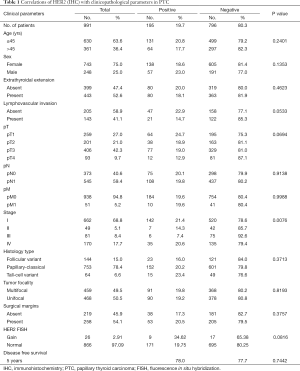
The details of 1,040 patients selected for analyses are as follows. The mean age of the patients at initial surgery was 40.4 years (range, 6–92 years), and 261 were (25.1%) males and 779 (74.9%) were females. The mean duration of follow-up was 76.5 months (range, 0–280 months). Seven hundred and ninety one (78.3%) of the tumours were classical papillary carcinomas; 153 (15.1%) were the follicular variant of PTC; and 66 (6.5%) were the tall cell variant. Extrathyroidal extension was seen in 462 (52.9%) cases and AJCC staging was as follows: 693 (68.6%) stage I; 51 (5.1%) stage II; 84 (8.3%) stage III; and 182 (18.0%) stage IV. Details of surgical margin status were available in only 490 cases and involved surgical margins were noted in 266 (54.2%) cases.
HER2 expression
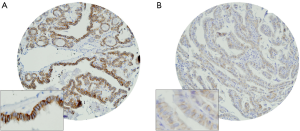
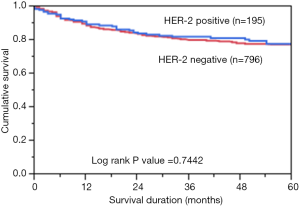
Immunohistochemical analysis of HER2 expression was interpretable in 991 PTC spots. While 796 cancers (80.3%) showed negative HER2 staining (score 0 and 1), there were 363 with score 0, 433 (43.7%) with 1+, 194 (19.6%) with 2+, and 1 (0.1%) with 3+ immunostaining. Total number of cases which demonstrated overexpression of 2+/3+ were 195 (19.7%). Representative cases are shown in Figure 1. HER2 positivity (2+/3+) in PTC was significantly associated with early stage tumors (Stage I) (P=0.0076). However, HER2 expression was not associated with age, gender, lymphovascular invasion or extrathyroidal extention (Table 1). There was no difference in survival between patients showing variable HER2 protein expression (P=0.7442) (Figure 2).
Full table
HER2 FISH
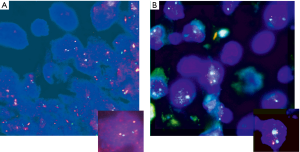
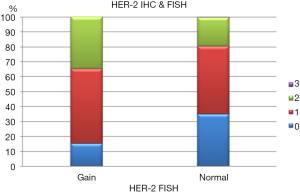
HER2 by FISH was interpretable in 913 PTC spots. Amplification was not seen in any of our cases and the incidence of HER2 gain in our cohort was only 3% (26 of 913) of cases. Tumors with representative FISH findings are shown in Figure 3. There was a tendency towards higher protein expression levels in tumors with a HER2 gene copy gain as compared to cancers with normal HER2 gene copy status, but this association did not reach statistical significance (P=0.0816) (Figure 4). No association was seen between HER2 FISH gain and tumor phenotype or patient survival.
Discussion
Our analyses revealed a 2+ HER2 staining in 19.6% and a 3+ HER2 staining in only 1 (0.1%) of our 991 interpretable tumors. This is in the range of earlier studies that were also done using FDA approved HER2 detection kits. Mdah et al. found 8.6% 2+ positive and 6.9% 3+ positive cases in a series of 58 papillary thyroid cancers (20). Sugishita et al. found 46% 2+ positive and 38% 3+ positive cases in a series of 37 papillary thyroid cancers (17). Mondi et al. found 33% 1+ positive however, no 2+ or 3+ positive cases, in a set of six papillary thyroid cancers (19). The reported data on HER2 expression were more variable in earlier studies using other non-FDA approved reagents. Here, Haugen et al. found 78% HER2 positive cases in 14 papillary thyroid cancers (22), Balta et al. observed 14.9% HER2 positive cases in 47 papillary thyroid cancers (16), Wu et al. identified 79.5% HER2 positive cases in 331 papillary thyroid cancers (25) and, Utrilla et al. described 52% HER2 positive cases in 25 papillary thyroid cancers (26).
Due to the high prevalence of papillary thyroid cancer in Saudi Arabia we were able to collect a cohort of patients that is substantially larger than available for comparable studies evaluating potential prognostic biomarkers. TMA studies on PTCs have so far included 331 tumors (25). High number of cases are imperatively needed for analyzing the possible role of biomarkers in the context of tumor aggressiveness, as only few papillary thyroid cancers show a dismal clinical course with distant metastases and/or recurrent disease after radio-iodine therapy. Tumor specific life expectancy was 89.9% after 5 years and 86.2% after 10 years in a register based study on 2,729 patients with papillary thyroid cancer from Sweden (27). This is comparable to our cohort with a survival rate of over 90% after 5 years (28).
The lack of unequivocal associations of HER2 expression with parameters of malignancy such as extrathyroidal tumor expansion, pT category, UICC stage, nodal (pN) or distant metastasis (pM), and tumor recurrence, demonstrates that HER2 expression is not a parameter of poor prognosis in papillary thyroid cancer. This is different from breast cancer, where HER2 expression is strongly linked to high tumor aggressiveness in patients that are not treated with anti-HER2 drugs (29-33).
The difference in the impact of HER2 expression may be caused by variations in the molecular environment of thyroid and breast cancer cells or just be due to different HER2 expression levels. Papillary thyroid cancers mostly exhibit a 2+ HER2 expression while breast cancers have a 3+ expression caused by high level gene amplification in most cases (34-39). The absence of a prognostic impact of HER2 expression in our study is in line with several earlier studies evaluating papillary thyroid cancers and also finding no association with tumor stage (16-19), metastasis (16,17,19,20), tumor subtype (16-20) and extrathyroidal extension (17,19).
The utility of HER2 as a therapeutic target has particularly been demonstrated for breast cancers showing unequivocal HER2 amplification with a HER2/centromere 17 ratio that usually markedly exceeds the threshold value of 2.0. Although the discussion continues on whether high level HER2 expression can occur in the absence of amplification and whether 2+ overexpression, caused by high polysomy can also result in some response to anti-HER2 therapy. After more than 15 years of clinical experience with anti-HER2 therapy, it appears that such events are not frequent or evident (40). Based on the experience in breast cancer, it thus seems unlikely that papillary thyroid cancer will be a good candidate for anti-HER2 therapy.
In our study, most HER2 positive cancers only showed 2+ positivity, exclusive of one 3+ case, with no cases even borderlining HER2 gene amplification. This is in line with data from Mondi et al. (19) and Mdah et al. who showed 6.9% HER2 overexpression in his patient cohort of 58 PTC and also failed to find amplification using chromogenic in situ hybridization (20). However, the study of Sugishita et al. suggested a HER2 amplification in 22% of cases (17). In this study, the cut-off level for amplification was selected at 1.3 instead of the typically used threshold of 2.0. None of the tumors in the 69 cases of Sugishita et al. had reached the cut-off level of 2.0. It is noteworthy, that a slight elevation of HER2 signals as compared to centromere 17 signals is mostly caused by allele duplication in the S- and G2 phases of the cell cycle which results in a visible duplication of the small HER2 signals but not of the much larger and confluent centromere signals (41).
Elevated HER2 copy numbers correlating with higher levels of protein expression was expected in our study. It is well known, that even a mildly increased gene copy number will often result in a mildly increased expression of the corresponding gene product (42). Even though a statistical significance was not obtained due to small number of cases, a clear tendency was indeed seen towards a higher protein expression in cancers with a HER2 gene copy number gain. Elevated protein expression measured by IHC has also been reported for EGFR family members in other cancers with increased gene copy numbers not meeting the formal criteria for gene amplification types (43,44).
Conclusions
Our results demonstrated that mild HER2 overexpression occurs at relevant frequency in papillary thyroid cancer and in the absence of gene amplification. In conclusion, expression of HER2 seems to hold no value as a prognostic factor in PTC. It appears possible, however, that next generation anti-HER2 drugs that may target even lower level HER2 expressing cancer cells could have an effect on a subset of papillary thyroid cancers.
Acknowledgments
We thank Sandeep Kumar Parvathareddy, Sarah Siraj, Valorie Balde, Mary Joan Galvez, Felisa De Vera, Hassan Al-Dossari, Padmanaban Annaiyappanaidu and Zeeshan Qadri, for technical assistance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.37). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board of the King Faisal Specialist Hospital and Research Centre approved the study under Project RAC# 2080-031 on PTC archival clinical samples. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bazarbashi S: Cancer Incidence report Saudi Arabia, 2010. Available online: http://www.chs.gov.sa/Ar/mediacenter/NewsLetter/2010%20Report%20(1).pdf
- Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55:10-30. [Crossref] [PubMed]
- Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol) 2010;22:395-404. [Crossref] [PubMed]
- Schlumberger M, Baudin E, Travagli JP. Papillary and follicular cancers of the thyroid. Presse Med 1998;27:1479-81. [PubMed]
- Sugitani I, Fujimoto Y. Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr J 1999;46:209-16. [Crossref] [PubMed]
- May FE. Novel drugs that target the estrogen-related receptor alpha: their therapeutic potential in breast cancer. Cancer Manag Res 2014;6:225-52. [Crossref] [PubMed]
- Bilici A. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol 2014;20:3905-15. [Crossref] [PubMed]
- Jin Y, Li Y, Pan L. The target therapy of ovarian clear cell carcinoma. Onco Targets Ther 2014;7:1647-52. [Crossref] [PubMed]
- Nguyen KS, Neal JW, Wakelee H. Review of the current targeted therapies for non-small-cell lung cancer. World J Clin Oncol 2014;5:576-87. [Crossref] [PubMed]
- Allgayer H, Babic R, Gruetzner KU, et al. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol 2000;18:2201-9. [Crossref] [PubMed]
- Zhang X, Silva E, Gershenson D, et al. Amplification and rearrangement of c-erb B proto-oncogenes in cancer of human female genital tract. Oncogene 1989;4:985-9. [PubMed]
- Yokota J, Yamamoto T, Toyoshima K, et al. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet 1986;1:765-7. [Crossref] [PubMed]
- Hall PA, Hughes CM, Staddon SL, et al. The c-erb B-2 proto-oncogene in human pancreatic cancer. J Pathol 1990;161:195-200. [Crossref] [PubMed]
- D’Emilia J, Bulovas K, D’Ercole K, et al. Expression of the c-erbB-2 gene product (p185) at different stages of neoplastic progression in the colon. Oncogene 1989;4:1233-9. [PubMed]
- Burandt E, Schreiber M, Stein A, et al. Continuous tissue microarray based identification of cancers with homogeneous target expression for successful targeted therapy in clinical routine practice. Genes Chromosomes Cancer 2014;53:228-39. [Crossref] [PubMed]
- Balta AZ, Filiz AI, Kurt Y, et al. Prognostic value of oncoprotein expressions in thyroid papillary carcinoma. Med Oncol 2012;29:734-41. [Crossref] [PubMed]
- Sugishita Y, Kammori M, Yamada O, et al. Amplification of the human epidermal growth factor receptor 2 gene in differentiated thyroid cancer correlates with telomere shortening. Int J Oncol 2013;42:1589-96. [PubMed]
- Kremser R, Obrist P, Spizzo G, et al. Her2/neu overexpression in differentiated thyroid carcinomas predicts metastatic disease. Virchows Arch 2003;442:322-8. [PubMed]
- Mondi MM, Rich R, Ituarte P, et al. HER2 expression in thyroid tumors. Am Surg 2003;69:1100-3. [PubMed]
- Mdah W, Mzalbat R, Gilbey P, et al. Lack of HER-2 gene amplification and association with pathological and clinical characteristics of differentiated thyroid cancer. Mol Clin Oncol 2014;2:1107-10. [PubMed]
- Lemoine NR, Wyllie FS, Lillehaug JR, et al. Absence of abnormalities of the c-erbB-1 and c-erbB-2 proto-oncogenes in human thyroid neoplasia. Eur J Cancer 1990;26:777-9. [Crossref] [PubMed]
- Haugen DR, Akslen LA, Varhaug JE, et al. Expression of c-erbB-2 protein in papillary thyroid carcinomas. Br J Cancer 1992;65:832-7. [Crossref] [PubMed]
- Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844-7. [Crossref] [PubMed]
- Liu YH, Xu FP, Rao JY, et al. Justification of the change from 10% to 30% for the immunohistochemical HER2 Scoring criterion in breast cancer. Am J Clin Pathol 2009;132:74-9. [Crossref] [PubMed]
- Wu G, Wang J, Zhou Z, et al. Combined staining for immunohistochemical markers in the diagnosis of papillary thyroid carcinoma: improvement in the sensitivity or specificity? J Int Med Res 2013;41:975-83. [Crossref] [PubMed]
- Utrilla JC, Martín-Lacave I, San Martín MV, et al. Expression of c-erbB-2 oncoprotein in human thyroid tumours. Histopathology 1999;34:60-5. [Crossref] [PubMed]
- Lundgren CI, Hall P, Ekbom A, et al. Incidence and survival of Swedish patients with differentiated thyroid cancer. Int J Cancer 2003;106:569-73. [Crossref] [PubMed]
- Siraj AK, Bavi P, Abubaker J, et al. Genome-wide expression analysis of Middle Eastern papillary thyroid cancer reveals c-MET as a novel target for cancer therapy. J Pathol 2007;213:190-9. [Crossref] [PubMed]
- Buza N, Roque DM, Santin AD. HER2/neu in Endometrial Cancer: A Promising Therapeutic Target With Diagnostic Challenges. Arch Pathol Lab Med 2014;138:343-50. [Crossref] [PubMed]
- Kim M, Agarwal S, Tripathy D. Updates on the treatment of human epidermal growth factor receptor type 2-positive breast cancer. Curr Opin Obstet Gynecol 2014;26:27-33. [Crossref] [PubMed]
- O'Sullivan CC, Swain SM. Pertuzumab: evolving therapeutic strategies in the management of HER2-overexpressing breast cancer. Expert Opin Biol Ther 2013;13:779-90. [Crossref] [PubMed]
- English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol Diagn Ther 2013;17:85-99. [Crossref] [PubMed]
- Cervera P, Fléjou JF. Changing pathology with changing drugs: tumors of the gastrointestinal tract. Pathobiology 2011;78:76-89. [Crossref] [PubMed]
- Van Bockstal M, Lambein K, Denys H, et al. Histopathological characterization of ductal carcinoma in situ (DCIS) of the breast according to HER2 amplification status and molecular subtype. Virchows Arch 2014;465:275-89. [Crossref] [PubMed]
- Lee AH, Key HP, Bell JA, et al. The effect of delay in fixation on HER2 expression in invasive carcinoma of the breast assessed with immunohistochemistry and in situ hybridisation. J Clin Pathol 2014;67:573-5. [Crossref] [PubMed]
- Curtit E, Nerich V, Mansi L, et al. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. Oncologist 2013;18:667-74. [Crossref] [PubMed]
- Crocetti E, Caldarella A, Ferretti S, et al. Consistency and inconsistency in testing biomarkers in breast cancer. A GRELL study in cut-off variability in the Romance language countries. Breast 2013;22:476-81. [Crossref] [PubMed]
- Makroo RN, Chowdhry M, Kumar M, et al. Correlation between HER2 gene amplification and protein overexpression through fluorescence in situ hybridization and immunohistochemistry in breast carcinoma patients. Indian J Pathol Microbiol 2012;55:481-4. [Crossref] [PubMed]
- Petroni S, Addati T, Mattioli E, et al. Centromere 17 copy number alteration: negative prognostic factor in invasive breast cancer? Arch Pathol Lab Med 2012;136:993-1000. [Crossref] [PubMed]
- Pauletti G, Godolphin W, Press MF, et al. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996;13:63-72. [PubMed]
- Sauter G, Moch H, Moore D, et al. Heterogeneity of erbB-2 gene amplification in bladder cancer. Cancer Res 1993;53:2199-203. [PubMed]
- Gremel G, Grannas K, Sutton LA, et al. In situ Protein Detection for Companion Diagnostics. Front Oncol 2013;3:271. [Crossref] [PubMed]
- Minner S, Rump D, Tennstedt P, et al. Epidermal growth factor receptor protein expression and genomic alterations in renal cell carcinoma. Cancer 2012;118:1268-75. [Crossref] [PubMed]
- Minner S, Jessen B, Stiedenroth L, et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res 2010;16:1553-60. [Crossref] [PubMed]


