
MET overexpression coexisting with epidermal growth factor receptor mutation influence clinical efficacy of EGFR-tyrosine kinase inhibitors in lung adenocarcinoma patients
Introduction
Lung cancer is the leading cause of cancer-related mortality in China (1,2). Epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) represent two most frequent targets in lung adenocarcinoma. Inhibitors targeting these two genes (erlotinib, gefitinib & afatinib for EGFR mutation; crizotinib & ceritinib for ALK rearrangements) have demonstrated promising clinical efficacies (3-6). Other genes, such as ROS1 and MET mutations, also benefited from targeted therapy (6-8).
Although most EGFR-mutant patients showed an excellent efficacy for epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) while 10–20% patients were non-responsive to EGFR-TKIs. T790M mutation and MET overexpression contributed to secondary resistance to EGFR-TKIs (7-12). Primary resistance is another challenge in clinical practice. Coexisting genetic alterations in cancer-driving genes were known to be associated with primary resistance for EGFR-TKIs (13,14). However, most studies focused on concurrent ALK and EGFR mutations (15,16). Other genes, such as MET overexpression and HER2 amplification were not well-known. Several studies demonstrated that around 20–50% NSCLC patients harbored MET overexpression (11). However, the frequency of concurrent EGFR and MET overexpression and the role of MET overexpression have remained unknown in EGFR-TKIs-treated EGFR-mutant patients. Additionally, it was previously shown that patients with MET overexpression had an inferior survival. However, its role has remained controversial in EGFR-mutant patients.
Here we evaluated the prevalence of MET overexpression and explored its efficacy for EGFR-TKIs in EGFR-mutant lung adenocarcinoma patients.
Methods
Patient selection
From January 2013 to July 2015, a total of 1,215 patients received EGFR mutation screening at Zhejiang Cancer Hospital. All EGFR mutations were confirmed by ARMS method (Amoy, Xiamen, China). Primary objective was to evaluate the impact of MET overexpression with concurrent EGFR mutation on the efficacy of EGFR-TKIs. And secondary objectives included frequency of concurrent MET overexpression in EGFR-mutant samples, overall survival (OS) and objective response rate (ORR), etc. Inclusion criteria included histologically-confirmed advanced stage, lung adenocarcinoma and a minimal age of 18 years. All EGFR-mutant patients received first-generation EGFR-TKIs. Those dying non-related with lung cancer were excluded. A total of 167 lung adenocarcinoma patients had sufficient samples for immunohistochemistry (IHC) for MET. The present study was approved by the Review Board of Zhejiang Cancer Hospital (IRB-2015-049).
MET IHC
IHC staining for MET overexpression was performed on 5 µm-thick FFPE tissue samples. Monoclonal MET primary antibody (Cell Signaling Technology, Danvers, MA,USA) was diluted with a factor of 1:500. Intensity score was defined as: 0= negative, 1= weak, 2= moderate or 3= strong positive. And the fraction of positive cells per intensity was estimated as a percentage. Four MET diagnostic subgroups were defined as: 3+ (≥50% of tumor cells stained with strong intensity); 2+ (≥50% of tumor cells with moderate or higher staining but <50% with strong intensity); 1+ (≥50% of tumor cells with weak or higher staining but <50% with moderate or higher intensity); or 0 (no staining or <50% tumor cells with any intensity). MET positivity was defined as a score of 2+ or 3+. IHC findings were analyzed by two independent specialists. IHC assay of MET overexpression was performed as previously described (15).
Efficacy evaluation
Tumor evaluations were performed every 8 weeks or earlier if there were significant signs of progression. And objective tumor responses were evaluated according to the scheme of Response Evaluation Criteria in Solid Tumors (RECIST 1.1).
Statistical analyses and follow-ups
Survival analysis was conducted with Kaplan-Meier method and intra-group differences were compared by log-rank test. Progression-free survival (PFS) of EGFR-TKI was defined as time from initiating EGFR-TKI therapy to documented progression or mortality from any cause. Statistical analysis was performed with SPSS 16 software (Chicago, IL, USA). P<0.05 was judged as statistical significance. And the last follow-up time was February 1, 2016.
Results
Patient characteristics
Among 167 EGFR-mutant patients, there were 95 males and 72 females with a median age of 59 years. All patients were conformed as having the histology of adenocarcinoma. There were 76 prior or current smokers and 91 non-smokers. Their clinical characteristics were summarized in Table 1.

Full table
MET overexpression
Among them, the mutations included deletion in exon 19 (n=81), L858R in exon 21 (n=72) and others (n=14). Fifty-seven samples were identified as MET positivity, including MET (3+) (n=19) and MET (2+) (n=38). No association existed between age, smoking, gender or EGFR mutation type. The clinical characteristics were compared between MET-positive and negative patients (Table 1).
Efficacy and survival outcomes
The response rate and disease control rate were 68.9% and 83.8% respectively. Among them, 81 patients with single EGFR mutation showed partial responses (73.6%) and stable disease (15.5%). And 9 patients had progressive disease. The response rate was 73.6% and disease control rate 91.8%. In EGFR/MET (+) patients, the response and disease control rates were 59.6% and 73.7% respectively. The efficacies of single EGFR-mutant and MET/EGFR (+) group were compared (Table 2).

Full table
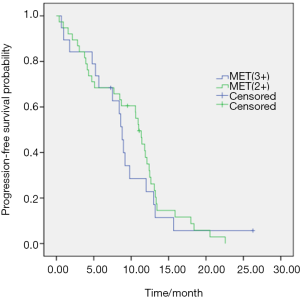
The median PFS was 10.8 months (95% CI, 9.7–11.9). And the values of PFS were 10.9 months (95% CI, 9.4–12.4) and 10.6 months (95% CI, 8.0–13.1) in single EGFR mutation and EGFR/MET (+) groups respectively (P=0.027) (Figure 1). No significant differences in PFS existed between patients with MET (2+) and MET (3+) (11.0 vs. 8.8 months, P=0.582) (Figure 2). There was a trend of higher ORR in patients with single EGFR mutations than that of EGFR/MET (+) alterations (73.6% vs. 59.6%, P=0.06). And a higher disease control rate was observed in single EGFR mutation patients than that of EGFR/MET (+) alterations (91.8% vs. 73.7%, P=0.0015).

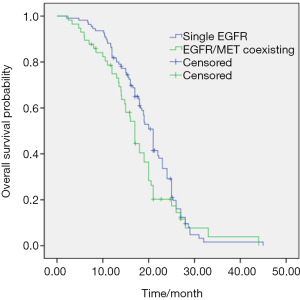
Sixty-seven (60.9%) and 38 (66.7%) patients received further chemotherapy in single EGFR and EGFR/MET (+) patients respectively. No other treatment type was observed in two groups. The median OS was 19.0 months (95% CI, 17.7–20.5). The values of OS were 21.0 and 17.0 months in single EGFR mutation and EGFR/MET (+) group respectively (P=0.078) (Figure 3).
Discussion
Our results demonstrated the frequency of MET overexpression in EGFR-mutant lung adenocarcinoma was 34.1%. EGFR-mutant patients with MET overexpression had a significantly shorter PFS and lower response rate for EGFR-TKIs.
With regards to MET pathway, previous studies focused mostly on amplification and exon mutation since both amplification and exon mutation might be targeted by inhibitors (15,16). As demonstrated by previous studies, the frequency of MET amplification was different between EGFR-TKI-naïve and EGFR-TKI-resistant NSCLC patients. The frequency of MET amplification was 2–3% in EGFR-TKI-naïve samples versus 5–20% in EGFR-TKI-resistant counterparts. As reported in the literature, the frequency of MET overexpression ranged from 20% to 50% (11,17). Antibodies, IHC techniques and evaluation criteria for MET positivity might contribute to frequency discrepancy between different studies (11). Furthermore cut-off values for high MET overexpression might also yield divergent results. In the present study, intensity score method was employed for evaluations. However, the “gold” method of evaluating MET expression must be validated by future multi-center studies.
With concurrent T790M mutation, MET overexpression induced acquired resistance of EGFR-TKIs. The percentages of MET/T790M (+) patients was 6.8% (14/207) and a combined use of EGFR-TKI and MET inhibitor showed a better efficacy in Gou et al. Study (18). Approximately 60–70% patients harboring EGFR mutation were responsive EGFR-TKIs and the range of PFS was 10 to 13 months (2-4). However, around 20% EGFR-mutant patients did not respond well to EGFR-TKIs. And this phenomenon was termed as primary resistance. Coexisting genetic alterations in cancer-driving genes, i.e., KRAS mutations, PTEN loss and BIM polymorphisms, were associated with primary resistance for EGFR-TKIs (19). The role of MET overexpression is currently ill-defined in non-EGFR-TKIs-treated patients. In the present study, concurrent MET and EGFR mutations could lower the clinical efficacy of EGFR-TKIs. Thus MET overexpression may be a factor of primary resistance.
There are some controversies of MET overexpression as a prognostic factor in NSCLC. As compared with MET (−) counterparts, there was a trend of unfavorable OS for MET (+) patients. Positive MET overexpression was an independent unfavorable prognostic factor in EGFR wild-type patients, but not in EGFR-mutant ones (20). Thus MET overexpression might play diverse roles in NSCLC harboring different gene alternations. In the present study, only a trend of OS difference existed between MET positive and negative patients. It was probably due to an imbalance of treatment options after ineffective EGFR-TKIs. And the prognostic value of MET expression should be further validated with future larger series.
There were some inherent limitations. The most prominent shortfall of our study lied in its small sample size and retrospective nature. Secondly, only one antibody and method were used for evaluating MET overexpression so that biases were inevitable. However, our findings are clinically relevant for EGFR-TKIs.
The coexistence of MET overexpression and EGFR mutation might influence the efficacy of EGFR-TKI. Prior to initiating EGFR-TKIs, the status of MET expression should be routinely detected. And future prospective studies with larger sample sizes are required for elucidating the clinical value of MET overexpression in EGFR-mutant patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study was approved by the Review Board of Zhejiang Cancer Hospital (IRB-2015-049) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe YWest Japan Oncology Group, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 2015;372:1700-9. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Zhang Y, Du Z, Zhang M. Biomarker development in MET-targeted therapy. Oncotarget 2016;7:37370-89. [PubMed]
- Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol 2016;34:794-802. [Crossref] [PubMed]
- Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of Non-small Cell Lung Carcinoma with poor prognosis. Clin Cancer Res 2016;22:3048-56. [Crossref] [PubMed]
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850-9. [Crossref] [PubMed]
- Guo B, Cen H, Tan X, et al. Prognostic Value of MET gene copy number and protein expression in patients with surgically resected non-small cell lung cancer: a meta-analysis of published literatures. PLoS One 2014;9:e99399 [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26 [Crossref] [PubMed]
- Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res 2011;17:1169-80. [Crossref] [PubMed]
- Tang Z, Du R, Jiang S, et al. Dual MET-EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant lung cancer. Br J Cancer 2008;99:911-22. [Crossref] [PubMed]
- Camidge DR, Ou SI, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32 suppl:abstr 8001.
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 Mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol 2016;34:721-30. [Crossref] [PubMed]
- Wu YL, Kim DW, Felip E, et al. Phase (Ph) II safety and efficacy results of a single-arm ph ib/II study of capmatinib (INC280) + gefitinib in patients (pts) with EGFR-mutated (mut), cMET-positive (cMET+) non-small cell lung cancer (NSCLC). J Clin Oncol 2016;34:abstr 9020.
- Gou LY, Li AN, Yang JJ, et al. The coexistence of MET over-expression and an EGFR T790M mutation is related to acquired resistance to EGFR tyrosine kinase inhibitors in advanced non-small cell lung cancer. Oncotarget 2016;7:51311-9. [PubMed]
- Oxnard GR, Arcila ME, Chmielecki J, et al. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res 2011;17:5530-7. [Crossref] [PubMed]
- Huang L, An SJ, Chen ZH, et al. MET Expression plays differing roles in non-small-cell lung cancer patients with or without EGFR mutation. J Thorac Oncol 2014;9:725-8. [Crossref] [PubMed]


