
Case report of a ganglioneuroma with metaplasia by adipocytes: use of a multi-disciplinary team as a key aid in difficult diagnosis and combined surgery
Introduction
Ganglioneuroma (GN) is a rare and well-differentiated benign tumor that arises from the sympathetic nervous system. It is slow-growing and occurs more commonly in children than in adults. GNs are most commonly found in the posterior mediastinum or the retroperitoneum. Most GNs have no symptoms and are found coincidentally on radiography during routine health examinations (1). GNs located in the posterior mediastinum and growing along the spine might penetrate the intervertebral foramen into the spinal canal. This modality is called a dumb-bell or sandglass tumor (2). The penetrating growth might cause damage to the vertebral body and the spinal cord. Although GNs are usually asymptomatic, complete resection is the optimal choice for a penetrating dumb-bell GN. Some complicated cases might require a variety of surgical techniques, thus benefitting from the participation of surgeons with various specialties (3).
Herein, we present a case of dumb-bell GN with metaplasia by adipocytes. The highlights in this case included not only the rare fatty replacement within the tumor, but also the location of the tumor, which resulted in uncertainty in diagnosis and preoperative planning.
Case presentation
A 24-year-old woman (height: 165 cm; weight: 52 kg) visited our department for examination of a right-sided posterior mediastinal mass that was incidentally discovered on a chest radiograph obtained during a routine medical examination. She was asymptomatic and had no significant past medical history. The results of a physical examination and laboratory tests were unremarkable, except for reduced air entry to her right lower chest.
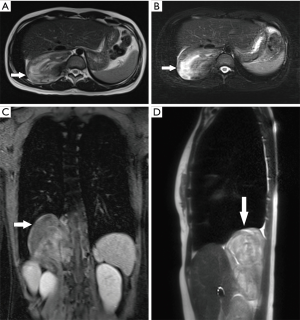
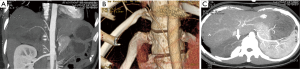
Non-contrast-enhanced and contrast-enhanced magnetic resonance imaging (MRI) of the thoracic and upper abdomen demonstrated a right paravertebral mass that extended from T10 to L1. The tumor was adjacent to and extended into the T10/T11 intervertebral foramen. The right kidney and the liver were partially displaced by the huge tumor. There were areas of heterogeneous density within the mass (Figure 1). Based on these radiologic features, the primary diagnosis was a neurogenic tumor in the posterior mediastinum or retroperitoneum. Because of the suspicion of malignancy, uncertainty of the tumor location, and the need to guide the subsequent surgery, computed tomography angiography (CTA) of the abdominal aorta was performed. The images revealed a mixed density tumor apparently adjacent to the thoracic vertebrae and the right posterior costophrenic angle. Non-contrast computed tomography (CT) images showed that the central portion of the tumor had an irregular strip of soft tissue density, with a CT value of −20 HU. Contrast-enhanced images confirmed the slightly heterogeneous tissue density observed on enhanced MRI. During the arterial phase, images showed blood vessels of the tumor originating from the posterior side of the abdominal aorta, the 11th intercostal artery, and the branches of the subcostal artery, thus nourishing the middle, upper, and lower parts of the tumor, respectively (Figure 2). In addition, the images showed that the 12th rib had been eroded owing to the infiltration and invasion by the tumor. Because the interior region of the tumor was rich in fatty tissues, an adipose tissue-derived tumor could not be excluded.
Multi-disciplinary team recruitment
After a brief discussion between thoracic surgeons and radiologists resulted in no clear consensus on the tumor location, general surgeons and neurosurgeons were called in for further discussion. Careful consideration was given to the planning of the surgical procedure. It was thought that the tumor was more likely to be located in the posterior mediastinum. However, the retroperitoneum could not be totally excluded as the tumor location. After discussion with the multi-disciplinary team (MDT), right-sided video-assisted thoracoscopic surgery (VATS) was considered as a first step. Next, if the tumor was located in the posterior mediastinum, thoracic surgeons and neurosurgeons would together complete the surgery. Otherwise, if the tumor was located in the retroperitoneum, general surgeons would cooperate with neurosurgeons to complete the surgery.
Resection
In preparation for surgery, the patient was placed in the left lateral decubitus position. A 1.5-cm long incision was made in the 8th intercostal space, along the anterior midaxillary line. Thoracoscopic exploration revealed that the tumor was located in the right posterior mediastinum and was close to the diaphragm. The diameter of the tumor was >10 cm. Sequential posterolateral thoracotomy was performed through the 10th intercostal space. The tumor was tightly adhered to the peripheral tissues and had a rich vascular supply. The main part of the rounded lesion was resected via careful blunt and sharp dissection. To expose the residual pedicels in the narrow foramen, neurosurgeons widened the T10/T11 foramen and carefully dissected and excised residual pedicles from the thoracic spine. Residual tumor tissues and pedicles on the surface of the dura mater were delicately dissected and removed. The mass had caused erosion in the 12th rib, and the entire rib was also resected.
Pathological findings
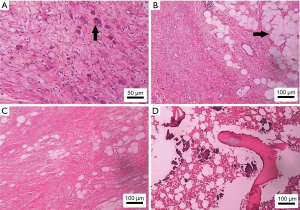
The tumor measured 12 cm × 10 cm × 5 cm, with a pedicle measuring 6.5 cm × 1.5 cm. Tumor sections were grayish-white in color and had a whorled pattern. Histopathological examination of the tumor showed that it comprised three cell types: spindle cells, ganglion cells, and adipose cells. Immunohistochemically, tumor cells were positive for S-100 spindle protein, vimentin, synaptophysin, and neuron specific enolase in focal areas, and were negative for CD68, neurofilament, desmosine, chromogranin A, and cytokeratin (Figure 3). The histopathological diagnosis of GN with metaplasia by adipocytes was definitively made after the discussion took place between two specialists.
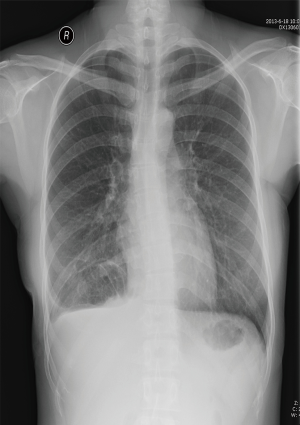
The patient has been undergoing regular follow-up examinations annually in our clinic. She shows no signs of recurrence 3 years since surgery (Figure 4).
Discussion
Nearly 75% of posterior mediastinal tumors originate from neurogenic tissues. GNs are tumors arising from the neural crest cells, and belong to a group that also includes peripheral nerve sheath tumors, melanomas, and neuroendocrine tumors (4). GNs are histologically different from schwannomas, neurofibromas, neurofibrosarcoma, pheochromocytomas, and other neurogenic tumors to some extent. These other tumors originate from sympathetic ganglion cells, peripheral nerves, and paraganglia (5). Separate mature ganglion cells in nests, Schwann cells in the stroma, neuroma, and fibrous tissue can be detected through microscopic examination of these tumors (6). GNs, as the rarest of neural crest tumors, are mainly found in adolescents or young adults (7). Schulman et al. reported that the mean age of presentation was 7 years (8). Patients with GNs are usually asymptomatic. However, patients might experience discomfort or pain in the chest, or have symptoms caused by intrusion into or compression of the spinal cord or other organs and tissues.
The extremely rare case presented here was that of a GN localized at the junction of the posterior mediastinum and the retroperitoneum in a young woman. The location led to a lack of consensus between the thoracic surgeons, general surgeons, neurosurgeons, and radiologists regarding the appropriate surgical treatment. The difficulty in surgical design was further complicated by the formation of a pedicle that extended from the tumor to the neural foramen. The appearance of fat cells within the GN was another unique feature in this case. GNs are typically composed of more benign and well-differentiated cells. It is unusual for GNs to have adipocyte metaplasia. Only a few such cases have been reported previously in English and Japanese (9-15). While the exact pathogenesis of a GN with adipocyte metaplasia is still unclear, we speculated that the atrophied tumor might be replaced gradually by mature adipocytes and adipose tissue (10). Alternatively, during metaplasia of the tumor, cells might be converted into adipocytes (14). In the current case, GN was not suspected until the surgical specimen was analyzed.
Radical resection of a dumb-bell GN is the optimal treatment choice. However, it is necessary to confirm and pinpoint the tumor location by CT and MRI. Evaluation of the tumor’s intraspinal invasiveness and extension based on CT and MRI are equally important in order to guide the surgical approach (16). On CT imaging, GNs might be identified as homogeneous or heterogeneous masses, with low to intermediate attenuation. Calcification can be detected in nearly 20% of cases. After contrast administration, the tumors demonstrate mild to moderate enhancement (17). On MRI, T1-weighted images display a hypointense lesion and T2-weighted images present a hyperintense lesion (18). In our case, MRI showed a homogeneous uniform signal on T1-weighted images and a heterogeneous hyperintense signal on T2-weighted images. Owing to the possibility of malignancy, CT and CTA of the tumor blood supply were performed to help guide the surgery. As in previous cases, our case showed a well-defined, heterogeneous lesion with an irregular strip of soft tissue (non-contrast CT value of -20 HU). Contrast-enhanced images demonstrated a slight heterogeneous enhancement. However, calcification in the tumor was not found. Blood supply to the tumor was confirmed in the arterial phase of a CTA scan and multiplanar reconstruction (MPR) of three-dimensional images from MRI and CTA scans. Additionally, erosion of the 12th rib by the tumor was detected.
The MPR images were analyzed by several radiologists to determine whether the tumor was located in the retroperitoneum or the posterior mediastinum, and to identify the exact blood supply to the mass. Unfortunately, there was no consensus about the precise location of the tumor. To facilitate decision-making for a surgical approach, a MDT was introduced. The MDT included experts from the departments of thoracic surgery, neurosurgery, general surgery, radiology, and pathology. The resulting agreement was to first confirm the location of the tumor by performing right-sided exploratory VATS through a 1.5-cm single port to minimize trauma. If the mass was determined to be located in the pleural cavity, the surgery would be completed by thoracic surgeons and neurosurgeons. General surgeons and neurosurgeons would finish the surgery if the tumor was located in the retroperitoneum. In this case, the tumor was in the pleural cavity. A MDT can facilitate the consensus opinion of all of the relevant specialists and other healthcare professionals before a management plan is undertaken (19).
Conclusions
GNs are tumors that arise from neural crest cells. A rare case of a GN with metaplasia by adipocytes has been presented. This type of dumb-bell tumor requires radical resection because it can cause chest pain and severe compression symptoms as the disease progresses. MPR can be used to observe the tumor in axial, sagittal, and coronal planes, so that the location of the tumor and its relationship with adjacent organs and tissues can be determined. VATS exploration is an effective way to intraoperatively evaluate the precise location of the tumor before resection. During the perioperative period, the recruitment of MDT resources is invaluable for assessing and formulating individual surgical plans.
Acknowledgments
We appreciate the invaluable assistance of Prof. Ci Li (Department of Pathology, the Affiliated Hospital of Chengdu University) in the histopathological diagnosis and Prof. Yun-Tao Chen (Department of Radiology, the Affiliated Hospital of Chengdu University) in the radiological diagnosis of the ganglioneuroma.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.67). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Erem C. Ganglioneuroma: An Overview. Tumors of the Central Nervous System 2012;9:137-46.
- Akwari OE, Payne WS, Onofrio BM, et al. Dumbbell neurogenic tumors of the mediastinum. Diagnosis and management. Mayo Clin Proc 1978;53:353-8. [PubMed]
- Rzyman W, Skokowski J, Wilimski R, et al. One step removal of dumb-bell tumors by postero-lateral thoracotomy and extended foraminectomy. Eur J Cardiothorac Surg 2004;25:509-14. [Crossref] [PubMed]
- Haithcock BE, Zagar TM, Zhang L. Diseases of the Pleura and Mediastinum. In: Abeloff’s Clinical Oncology. 5rd edition. Philadelphia: Churchill Livingstone 2014:1193-206.e4.
- Kızıldağ B, Alar T, Karatağ O, et al. A case of posterior mediastinal ganglioneuroma: the importance of preoperative multiplanar radiological imaging. Balkan Med J 2013;30:126-8. [Crossref] [PubMed]
- Pandiyan MS, Cherian VK, Christopher DJ. Chest wall mass with double pathology. Eur J Cardio Thorac 2006;29:625-6. [Crossref] [PubMed]
- Moriwaki Y, Miyake M, Yamamoto T, et al. Retroperitoneal ganglioneuroma: a case report and review of the Japanese literature. Intern Med 1992;31:82-5. [Crossref] [PubMed]
- Schulman H, Laufer L, Barki Y, et al. Ganglioneuroma: an 'incidentaloma' of childhood. Eur Radiol 1998;8:582-4. [Crossref] [PubMed]
- Zhang Y, Nishimura H, Kato S, et al. MRI of ganglioneuroma: histologic correlation study. J Comput Assist Tomogr 2001;25:617-23. [Crossref] [PubMed]
- Hara M, Ohba S, Andoh K, et al. A case of ganglioneuroma with fatty replacement: CT and MRI findings. Radiat Med 1999;17:431-4. [PubMed]
- Ko SM, Keum DY, Kang YN. Posterior mediastinal dumbbell ganglioneuroma with fatty replacement. Br J Radiol 2007;80:e238-40. [Crossref] [PubMed]
- Duffy S, Jhaveri M, Scudierre J, et al. MR imaging of a posterior mediastinal ganglioneuroma: fat as a useful diagnostic sign. AJNR Am J Neuroradiol 2005;26:2658-62. [PubMed]
- Hasegawa A, Kato K, Yamamoto H, et al. A case of posterior mediastinal ganglioneuroma with fat tissue. Nihon Kokyuki Gakkai Zasshi 2001;39:792-6. [PubMed]
- Adachi S, Kawamura N, Hatano K, et al. Lipomatous ganglioneuroma of the retroperitoneum. Pathol Int 2008;58:183-6. [Crossref] [PubMed]
- Yorita K, Yonei A, Ayabe T, et al. Posterior mediastinal ganglioneuroma with peripheral replacement by white and brown adipocytes resulting in diagnostic fallacy from a false-positive 18F-2-fluoro-2-deoxyglucose-positron emission tomography finding: a case report. J Med Case Rep 2014;8:345. [Crossref] [PubMed]
- Rha SE, Byun JY, Jung SE, et al. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics 2003;23:29-43. [Crossref] [PubMed]
- Ichikawa T, Ohtomo K, Araki T, et al. Ganglioneuroma: computed tomography and magnetic resonance features. Br J Radiol 1996;69:114-21. [Crossref] [PubMed]
- Kato M, Hara M, Ozawa Y, et al. Computed tomography and magnetic resonance imaging features of posterior mediastinal ganglioneuroma. J Thorac Imaging 2012;27:100-6. [Crossref] [PubMed]
- Powell HA, Baldwin DR. Multidisciplinary team management in thoracic oncology: more than just a concept? Eur Respir J 2014;43:1776-86. [Crossref] [PubMed]


