Plasma thioredoxin reductase activity, a diagnostic biomarker, is up-regulated in resectable non-small cell lung cancers
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. The incidence rate of lung cancer is the second highest, but the mortality is highest among all malignant tumors (1). Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases. Most NSCLC patients are diagnosed at an advanced stage and have a five-year overall survival rate of less than 5% (2). The poor survival rate strongly suggests the significance of the development of early diagnostic techniques. However, until now, none of the tumor-associated biomarkers has been proven to be effective for early diagnosis in NSCLC patients.
Thioredoxin reductase (TrxR/TR) is a member of the selenium-containing pyridine nucleotide-disulfide oxidoreductase family and consists of three isoforms, TR1, TR2 and TR3. TR1 is the major isoform and is localized in the cytoplasm, where it catalyzes the NADPH-dependent reduction of oxidized thioredoxin (Trx). The TR system has been well-documented to play an essential role in various metabolic pathways including selenium metabolism, DNA synthesis, and oxidation resistance in cancer cells (3,4). Over-expression of TR1 has been observed in multiple carcinoma cell lines and tissues (5,6). In human breast and hepatocellular cancer, over-expression of TR1 has been proven to be an independent marker for a poor prognosis in patients (7,8). Elevated TR activity was observed in men at a high risk for prostate cancer (9).
Previous studies have shown that depletion of TR1 can reverse the tumorigenicity of lung carcinoma cells (10). Numerous studies have further validated the anti-tumor effect of TR1-specific inhibitors (11-15). Our group has demonstrated that 1,2-[bis (1,2-Benzisoselenazolone-3 (2H)-ketone)] ethane (BBSKE), a specific inhibitor of TR, was able to induce carcinoma cell apoptosis, and the mechanism for this effect involved the suppression of the TrxR/TR-Tr-NF-kappaB pathway (16-18). The biological mechanism for TR in cancer proliferation suggested the natural hypothesis that TR is a diagnostic biomarker. Thus, it was of great clinical significance to develop a technically convenient, economically beneficial and scientifically reproducible method for the early diagnosis of NSCLC.
Methods
Study design and patients
This was a prospective, observational clinical study that consisted of two parts, a “development cohort” and a “validation cohort.”
In the “development cohort”, blood samples were collected from 309 patients who had been diagnosed with malignant tumors by pathological analysis in three hospitals (Hubei Renmin Hospital, Wuhan Zhongnan Hospital and Hunan Cancer Hospital) and 667 healthy people in four medical centers (Beijing, Wuhan, Guangzhou and Changsha) in China. The plasma TR activity was tested, and receiver operating characteristic (ROC) curve analysis was then performed to determine the diagnostic value of TR activity.
In the “validation cohort”, patients who met the following criteria were eligible: (I) 18–80 years old; (II) clinically diagnosed with resectable NSCLC, according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging for NSCLC: T1–3, N0–2, M0 or T4, N0–1, M0; (III) anti-tumor treatment-naïve. Informed consent was signed by each patient for the scientific use of the biological samples. This study was approved by the Ethics Committee of the Hunan Cancer Hospital and registered on Clinicaltrials.gov (NCT01985113). The biological specimens were recorded in the tissue bank of the Precision Medical Center of Cancer of the Affiliate Cancer Hospital of Xiangya School of Medicine Central South University. The plasma TR activity was measured to confirm the diagnostic cut-off value in the development cohort.
Sample preparation
Blood samples were drawn on the day before surgery and 3–5 days after surgery and stored at 4 °C. Centrifugation (4 °C, 3,000 rpm for 5 min) was performed within 8 hours. The plasma samples were stored at −80 °C before the test. Paired lung carcinoma and adjacent normal tissues (10 mm outside of the tumor border) were cut into 5 mm × 5 mm pieces and then immediately stored in liquid nitrogen. The total tissue protein was isolated using RIPA Lysis Buffer (Applygen Technologies Inc.), and the protein concentration was determined using the Bradford assay (Applygen Technologies Inc.).
Measurement of TR activity in plasma/tissue using DTNB assay
We utilized the NADPH-dependent DTNB reduction assay to measure the plasma and tissue TR activities (Keaise TR Detection Kit, No. 3400264 2014, approved by China Food & Drug Administration). The reaction equation is as follows:
First, a stock mixture (10 mM EDTA-Na2, 5 mM DTNB, 0.2 mM NADPH, and 0.2 mg/mL bovine serum albumin in 100 mM PBS, pH 7.0) was freshly prepared and stored at 4 °C. For the analyses, 15 µL of plasma or 20 µg of protein was mixed with 20 µL of the TR inhibitor Ethaselen (BBSKE, 0.5 µM) or 20 µL of PBS, added to a 96-well plate, and incubated at 37 °C for 30 min. The reactions were started by the addition of 165 µL of the stock mixture to each sample. The changes in the absorbance at 405 nm with time were monitored for the first 7 min at 37 °C using a microplate reader. The TR activity was calculated as follows: TR activity = ΔA412 nm / min × sample dilution × total volume / 6.35 mM−1cm−1 / plasma volume or protein content. The TR activities of the plasma and tissue were expressed as U/mL or U/mg, respectively.
Performance evaluation of TR detection assay
Limit of detection (LOD)
Standard samples (rat liver TR: 3.48, 1.74, 0.87, 0.65, or 0.43 U/mL) or empty buffer were used in the TR detection assay for technical standardization.
Sensitivity
0, 1, 3, 5, 10, 15, and 20 µL of rat liver TR (0.5 mg/mL) solution was added to the wells of a 96-well plate. The TR activity was tested using the Keaise TR Detection Kit. The sensitivity was calculated based on the standard curve generated using Origin 7.5.2 software.
Accuracy and reproducibility
Six and ten parallel determinations of TR activity were carried out using aliquots of a single control plasma sample (Wuhan Blood center, batch number 176985: group O, and negative for HBV, HCV, HIV and syphilis) to estimate the relative standard deviation and the coefficient of variation (CV), respectively.
Stability
The TR Detection Kit was stored in the dark at 37 °C for 7 days, and the sensitivity, accuracy and reproducibility tests were repeated.
CEA measurement
Whole blood was collected in an EDTA-anticoagulation tube, then centrifuged at 4 °C at 3,000 rpm for 5 min. The plasma was collected, and the samples were diluted into the sample diluent NS and assayed (Abcam, USA, 183365). A 50 µL aliquot of each sample was added to a 96-well plate, and 50 µL of the antibody cocktail was added to each well. The plate was incubated for 1 hour at room temperature, after which 100 µL of the stop solution was added to each well, and the optical density value was read at 450 nm using a Micro-plate reader (Tecan, USA, M1000pro).
Immunohistochemical analysis
The immunohistochemical analysis of carcinoma and para-carcinoma (adjacent normal tissues as described in the sample preparation section) tissues from the NSCLC patients was performed as described previously (19). The paired tissues were dewaxed in xylene (twice for 5 min) and rehydrated in a series of ethanol solutions (100%, 95%, 90%, 80%, 70%, 60%, 50%, 30%, and 3 times in ddH2O, each for 5 min). Antigen retrieval was performed in sodium citrate buffer (pH 6.0) in a boiling water bath for 10 min, and the samples were then allowed to cool at room temperature. The samples were then washed three times with PBS (5 min each time). After the endogenous peroxidase was blocked using a hydrogen peroxide solution (5 min), the blocking solution was removed, and the tissue sections were then incubated with the primary antibody to TR1 (Abcam, USA, 16840) at a dilution of 1:500 at 4 °C for 8 hours. After washing in phosphate-buffered saline (3 times for 5 min each time) and incubation with a secondary goat anti-rabbit IgG (Maxim, China, KIT-9710) for 30 min, staining was performed using 3,3’-Diaminobenzidine, and the nuclei were counterstained with hematoxylin. The TR1 expression was scored on the basis of both area and staining intensity. Positive cell counts less than 5%, 5–25%, 25–50%, 50–75% and 75–100% were defined as 0, 1, 2, 3 and 4, respectively. The intensity of the cell staining was evaluated as colorless, light yellow, tawny and brown and assigned scores of 0, 1, 2 and 3, respectively. These two scores were added together and defined as follows: 0–1 (−), 2–3 (+), 4–5 (++), and 6–7 (+++). Positive was defined as ++/+++.
Cell culture
The human lung adenocarcinoma cell line, A549, was purchased from American Type Culture Collection. The cells were cultured in Roswell Park Memorial Institute 1640 with 10% fetal bovine serum (Gibco, USA, 10099-141) at 37 °C in a 5% CO2 atmosphere.
CCK-8 testing
After the A549 cells were cultured for 24 to 48 hours and the cells were in a logarithmically increasing phase, the cells were counted and diluted to 1×104/100 µL, then cultured in 96-well plates. After 24 hours, 11 µL of BBSKE solution (50 or 100 µM) were added to each of six replicate wells, and the cells were cultured for 24 or 48 hours longer. Finally, 10 µL of the CCK-8 solution was added to each well and cultured in 37 °C for 1.5 hours, and the optical density value was read at 450 nm using a Micro-plate reader (Tecan, USA, M1000pro).
Flow cytometry
A549 cells were treated with one of two concentrations of BBSKE or DMSO for 24 hours and then harvested for the apoptosis test (Becton, Dickinson and Company, USA, #556547). The cells were washed with 3 times with PBS then resuspended in 200 µL binding buffer to which 5 µL of Annexin V was added. The samples were placed in the dark for 10 min then labeled with 10 µL of PI, then immediately analyzed using flow cytometry (Becton, Dickinson and Company, USA, FACSCalibur).
Western blotting
Western blotting was performed as previously described (20). The primary antibodies were as follows, Anti-NF-kappaB p65 antibody (Abcam, USA, 16502), phospho-p65 (Abcam, USA, #86299), TR1 (Abcam, USA, #16840) and β-actin (Abcam, USA, #8226). The Western blot band intensities were read using a Bio-Rad ChemiDocTM MP Imaging system.
Nude mice xenografts
Four-week-old 16–18 g male BALB/c nude mice were ordered from an animal-supply company (Shanghai) and raised in an animal center under specific pathogen-free conditions. Logarithmic phase A549 cells were diluted to 5×106/200 µL and then injected subcutaneously. After the tumor size was larger than 100 mm3, BBSKE was administered by gavage at one of two doses (36 or 72 mg/kg) for 12 days. Alternatively, cis-platinum (2.5 mg/kg) was given by intraperitoneal injection on the first treatment day.
Statistical analysis
The SPSS 21.0 software package was used to perform the statistical analyses (SPSS Institute, version 21.0, Chicago, USA). Because the TR values demonstrated a non-normal distribution, this parameter could not be expressed as the mean value and standard deviation. The TR values are therefore expressed as the medians and interquartile range (IQR). The statistical significance of the differences in the plasma TR activities between groups was determined using logistic regression. ROC curves were generated to evaluate the performance of TR activity in separating the cancer patients from the healthy individuals. All tests were two-tailed, and P values <0.05 were considered to be statistically significant.
Results
Performance evaluation of TR detection assay
The prospective, two-part study design is shown in Figure 1. A total of 976 subjects were enrolled in the development cohort, and 192 patients were enrolled in the validation cohort. The baseline characteristics of all subjects including gender, tumor type and smoking history are shown in Table 1.
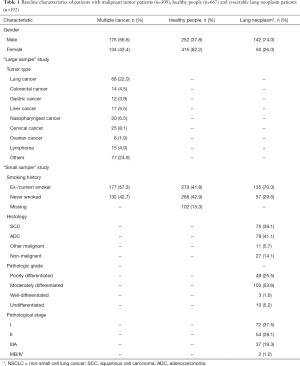
Full table
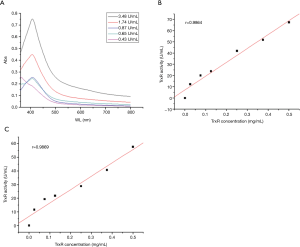
To evaluate the performance of the TR analysis assay, the LOD was measured using UV-VIS spectrophotometry of the results of a series of TR standard samples. The LOD was determined to be 0.65 U/mL, and the linear correlation coefficient of the curve-fit was 0.9864 (Figure S1). In the accuracy and reproducibility tests, the relative standard deviation and CV value in the same negative plasma sample were 7.22% and 14.71%, respectively.
Plasma TR activity in the development cohort
The plasma level of TR was measured in specimens from 309 cancer patients and 667 healthy people. The median TR value for the cancer patients was significantly higher than that for the healthy people, 9.85 vs. 0.65 U/mL, P<0.05.
Accuracy of the parameters for malignant tumor diagnosis by ROC analysis
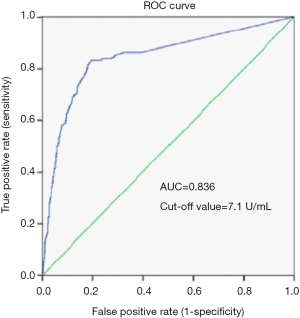
To assess the usefulness of the TR value in tumor diagnosis, an ROC curve analysis, which evaluates the true and false positive rates (sensitivity and 1-specificity), was performed (21), and the area under the curve (AUC) was calculated (Figure 2). According to the ROC curve analysis, the optimal threshold for separation of the plasma TR activity of the cancer patients (n=309) from that of the healthy people (n=667) was 7.1 U/mL, with 66.02% diagnostic sensitivity and 88.91% specificity (Figure 2); the AUC was 0.836.
TR activity and expression in NSCLC patients
After 7.1 U/mL was determined to be the threshold value in the development study, the diagnostic potential for TR was tested in the validation study.
Before surgery, the median TR value was significantly higher in the NSCLC patients (n=150) than that in the patients with benign diseases (n=27): 9.02 vs. 6.45 U/mL, P<0.05, respectively, which was consistent with the development study. In the NSCLC patients, the median TR was compared between males and females, smokers and non-smokers, squamous carcinoma and adenocarcinoma (chi-square), cancer stages I–IIA and IIB–IIIA, and no differences was detected (P>0.05). When the threshold value of TR (7.1 U/mL) was used to assess the NSCLC patients, the TR activity of 96 patients (64.0%) out of 150 was higher than 7.1 U/mL. After surgery, the median value of plasma TR decreased from 9.02 to 0.65 U/mL.
The decrease of both TR and carcinoembryonic antigen (CEA) level after surgery
The CEA levels were measured in 150 NSCLC patients using ELISA (Table 2). The median CEA level before surgery was 2.42 ng/mL, and this fell to 1.39 ng/mL after surgery (P<0.05). A previous study suggested that CEA is a prognostic biomarker for NSCLC (22,23). A retrospective analysis showed that the serum CEA was positive in approximately 35.86% of NSCLC patients (24). However, CEA was not a diagnostic biomarker. In our study, the CEA levels of 53 subjects (35.3% sensitivity) were greater than 3.47 ng/mL. Additionally, the TR levels in 138 of the 149 NSCLCs patients (92.6%) decreased after surgery whereas 67.1% of the patients (100 out of 149) showed a decrease in the CEA level.

Full table
TR expression by IHC
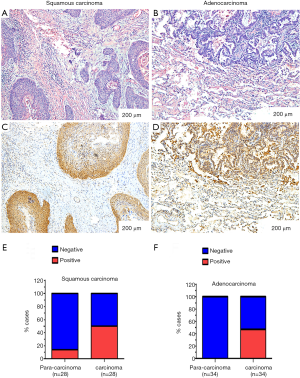
In this study, TR was determined to be positive (++/+++) in 48.4% (30/62) of the NSCLC patients. However, TR expression was rarely seen (1/17) in the specimens from patients with benign pulmonary diseases. The TR expression was predominantly located in the cytoplasm in both squamous cell carcinoma and adenocarcinoma tissues (Figure 3A-D), and the expression rates in squamous cell carcinoma and adenocarcinoma were comparable (Figure 3E,F): the TR expression rate was 50.0% (14/28) in squamous cell carcinoma and 47.1% (16/34) in adenocarcinoma.
A549 in vitro and in vivo studies using BBSKE
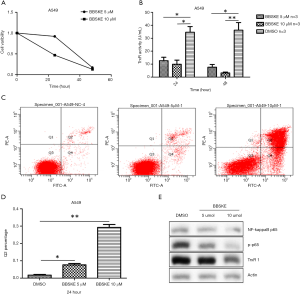
BBSKE is a small molecule agent that specifically inhibits TR. This compound was shown to inhibit TR activity and A549 cell proliferation in a dose-dependent manner in vitro (Figure S2A,B).
According to the CCK-8 assay, a 10 µM concentration of BBSKE inhibited the proliferation of the A549 cells by 53% at 24 hours and 87% at 48 hours (Figure S2A). The TR activity of the A549 cells was inhibited by BBSKE in a time- and dose-dependent manner. Specifically, in the presence of 5 µM BBSKE, the mean TR activity of the A549 cells was 12.7 U/mL at 24 hours and 7.6 U/mL at 48 hours, and at 10 µM, the activity was 9.9 and 3.2 U/mL at 24 and 48 hours, respectively, whereas the mean TR activity of the DMSO-treated cells was 34.6 and 36.2 U/mL at 24 and 48 hours, respectively (Figure S2B). As shown in Figure S2C, BBSKE induced A549 cell apoptosis in a dose-dependent manner at concentrations of 5 and 10 µM. The mean Q2 percentages for cells treated with DMSO, 5 µM BBSKE or 10 µM BBSKE were 1.7%, 7.6%, 29.3%, respectively, n=3 in Figure S2D. The Western blot analyses showed that the expression of NF-kappaB p65 was not affected by BBSKE whereas phosphor-p65 and TR1 were inhibited by BBSKE in a dose-dependent manner in Figure S2E.
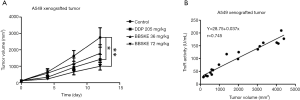
The A549 xenograft tumor burden was inhibited by DDP and BBSKE compared with NS (Figure S3A). None of 20 nude mice died after 12 days of continuous BBSKE treatment. There was no statistically significant difference in the body weights among the various treatment groups. The mean tumor volumes were: DMSO group 2835±503 mm3 (SD); DDP group, 1327±237 mm3; low-dose BBSKE group, 1,815±635 mm3; and high-dose BBSKE group, 1,109±211 mm3. The mean tumor volume was significantly smaller in the high-dose BBSKE group compared with the DDP group (P<0.05). In the in vivo study, the plasma TR activity of the tumor-bearing nude mice showed a positive correlation with the tumor volumes (Figure S3B).
Discussion
This was a prospective study to explore the diagnostic value of plasma TR in cancer patients. In this two-step study, the cut-off value (7.1 U/mL) was determined by the development study, and the diagnostic value (7.1 U/mL) was verified in the validation study in NSCLC patients.
In the development study, the median TR value of the cancer patients was significantly higher than that of healthy people, 9.85 vs. 0.65 U/mL, P<0.05. ROC curve analysis can determine an efficient diagnostic value and designate the optimal threshold of the TR value. This analysis was therefore performed in the development study. According to the ROC curve analysis, the sensitivity was 66.02% and the specificity was 88.91% at the cut-off value (7.1 U/mL). These results showed that an elevation in TR was rigorously consistent with malignancy. First, the plasma TR activity in the cancer patients was significantly higher than that in the healthy people in the development study. Second, the TR values were higher in the lung cancer patients than the patients with benign diseases in the validation study. In addition, the TR activity in the carcinoma tissues was higher than that in para-carcinoma tissues in the pathology specimens from the patients diagnosed with NSCLC in the validation study. Furthermore, an elevation in TR was consistent with the previous study in men at high risk for prostate cancer (9). The results in the development and validation studies proved that TR was generally elevated in cancer patients as well as in NSCLC patients.
The cut-off value generated in large sample study was further verified in NSCLC patients. Before surgery, the TR value exceeded 7.1 U/mL in 64.0% (96/150) of the NSCLC patients. The diagnostic sensitivity (64.0%) was comparable to that (66.02%) in the development study. In addition, the TR value was dramatically decreased after tumor excision. This decline in the TR value in the peripheral blood was consistent with the observations in a study of hepatocellular carcinoma (8), which suggested that TR might be a cancer prognostic biomarker. However, further follow-up investigations of the NSCLC patients are urgently needed to study the diagnostic value of TR.
After the diagnostic value of TR was verified in NSCLC patients, further studies to demonstrate the biological connection between TR and carcinoma were performed. First, TR expression was evaluated in the surgical specimens of the NSCLC patients. TR expression (++/+++) was detected in 48.4% of NSCLC patients but was rarely seen in para-carcinoma tissue or benign diseases. The expression of TR in NSCLC was comparable with that in multiple previously described forms of cancers (5,6). In our study, the plasma TR values were positively correlated with the tumor volumes in the tumor-bearing nude mice. Specifically, in the nude mice, when the tumor was larger, the TR value was higher, which supported the concept that the TR was released by cancer cells. A higher TR value was associated with a higher expression rate in cancer patients, which supported the concept that TR was associated with carcinoma disease status. However, in our study, TR expression was positive in one patient who was diagnosed with tuberculosis, which suggested that TR might not be highly specific to cancer.
Numerous studies have proven that TR plays a critical role in cancer genesis and cell proliferation (10-18,20,25). BBSKE is a specific TR inhibitor originally designed by our group (16). In this study, we repeated experiments in which A549 cells were treated with BBSKE in vitro and in vivo. These experiments showed that A549 cell proliferation was inhibited at dose- and time-dependent manner. In the A549 xenograft model, the 72 mg/kg dose of BBSKE demonstrated a better anti-cancer ability than DDP, and this was statistically significant compared to the NS control (Figure S3A). The BBSKE inhibition study demonstrated that TR was associated with carcinoma proliferation. However, the cell proliferation-promoting mechanism of TR is still unclear. Our previous study suggested that TR inhibition was associated with down-regulation of the NF-kappaB pathway (18). Previous studies have shown that NF-kappaB inhibitors could suppress cancer cell proliferation (26-28) and induce cancer cell apoptosis (29). In this study, phosphor-p65 expression was down-regulated by BBSKE in a dose-dependent manner, which was consistent with previous study (18). However, further study is needed to demonstrate the mechanism by which TR promotes proliferation. It was encouraging that BBSKE tablets have been approved for a phase 1 clinical trial by the China FDA for new drug investigation. This trial was registered on Clinicaltrials.gov (NCT02166242).
The main results of this study supported the concept that TR has a diagnostic value in NSCLC. Further studies to clarify the mechanism by which TR promotes cell proliferation and suppresses apoptosis are urgently needed.
Acknowledgments
The authors thank Keaise Medical Examination Center for technical assistance and TR detection, and thank Dr. Chunhua Zhou, Dr. Haiyan Yang, Dr. Wenjuan Jiang, Dr. Yi Xiong, Dr. Li Liu and Dr. Liang Zeng, Jie Li and Chunqin Shi for the clinical data collection and assistance with the follow-up visits.
Funding: This study was supported by the National Natural Science Foundation of China (grant No. 81372266 to H Zeng) and China Human Provincial Science & Technology Department (grant No. 14JJ2144 to N Yang, 2015JJ6064 to M Zhou).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.39). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was signed by each patient for the scientific use of the biological samples. This study was approved by the Ethics Committee of the Hunan Cancer Hospital and registered on Clinicaltrials.gov (NCT01985113). The biological specimens were recorded in the tissue bank of the Precision Medical Center of Cancer of the Affiliate Cancer Hospital of Xiangya School of Medicine Central South University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Mariotto AB, Noone AM, Howlader N, et al. Cancer survival: an overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr 2014;2014:145-86. [Crossref] [PubMed]
- Arnér ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol 2006;16:420-6. [Crossref] [PubMed]
- McEligot AJ, Yang S, Meyskens FL. Redox regulation by intrinsic species and extrinsic nutrients in normal and cancer cells. Annu Rev Nutr 2005;25:261-95. [Crossref] [PubMed]
- Lincoln DT, Ali EE, Tonissen KF, et al. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Res 2003;23:2425-33. [PubMed]
- Esen H, Feyzioglu B, Erdi F, et al. High thioredoxin reductase 1 expression in meningiomas undergoing malignant progression. Brain Tumor Pathol 2015;32:195-201. [Crossref] [PubMed]
- Cadenas C, Franckenstein D, Schmidt M, et al. Role of thioredoxin reductase 1 and thioredoxin interacting protein in prognosis of breast cancer. Breast Cancer Res 2010;12:R44. [Crossref] [PubMed]
- Li C, Peng Y, Mao B, et al. Thioredoxin reductase: a novel, independent prognostic marker in patients with hepatocellular carcinoma. Oncotarget 2015;6:17792-804. [Crossref] [PubMed]
- Karunasinghe N, Ferguson LR, Tuckey J, et al. Hemolysate thioredoxin reductase and glutathione peroxidase activities correlate with serum selenium in a group of New Zealand men at high prostate cancer risk. J Nutr 2006;136:2232-5. [PubMed]
- Yoo MH, Xu XM, Carlson BA, et al. Thioredoxin reductase 1 deficiency reverses tumor phenotype and tumorigenicity of lung carcinoma cells. J Biol Chem 2006;281:13005-8. [Crossref] [PubMed]
- Shao FY, Du ZY, Ma DL, et al. B5, a thioredoxin reductase inhibitor, induces apoptosis in human cervical cancer cells by suppressing the thioredoxin system, disrupting mitochondrion-dependent pathways and triggering autophagy. Oncotarget 2015;6:30939-56. [PubMed]
- Raninga PV, Di TG, Vuckovic S, et al. Inhibition of thioredoxin 1 leads to apoptosis in drug-resistant multiple myeloma. Oncotarget 2015;6:15410-24. [Crossref] [PubMed]
- Yan K, Lok CN, Bierla K, Che CM. Gold(I) complex of N,N'-disubstituted cyclic thiourea with in vitro and in vivo anticancer properties-potent tight-binding inhibition of thioredoxin reductase. Chem Commun (Camb) 2010;46:7691-3. [Crossref] [PubMed]
- Lu J, Chew EH, Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A 2007;104:12288-93. [Crossref] [PubMed]
- Marzano C, Gandin V, Folda A, et al. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic Biol Med 2007;42:872-81. [Crossref] [PubMed]
- Wang L, Yang Z, Fu J, et al. Ethaselen: a potent mammalian thioredoxin reductase 1 inhibitor and novel organoselenium anticancer agent. Free Radic Biol Med 2012;52:898-908. [Crossref] [PubMed]
- Zhao F, Yan J, Deng S, et al. A thioredoxin reductase inhibitor induces growth inhibition and apoptosis in five cultured human carcinoma cell lines. Cancer Lett 2006;236:46-53. [Crossref] [PubMed]
- Lan L, Zhao F, Wang Y, et al. The mechanism of apoptosis induced by a novel thioredoxin reductase inhibitor in A549 cells: possible involvement of nuclear factor-kappaB-dependent pathway. Eur J Pharmacol 2007;555:83-92. [Crossref] [PubMed]
- Kakolyris S, Giatromanolaki A, Koukourakis M, et al. Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res 2001;7:3087-91. [PubMed]
- Fu JN, Li J, Tan Q, et al. Thioredoxin reductase inhibitor ethaselen increases the drug sensitivity of the colon cancer cell line LoVo towards cisplatin via regulation of G1 phase and reversal of G2/M phase arrest. Invest New Drugs 2011;29:627-36. [Crossref] [PubMed]
- Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging 1989;29:307-35. [PubMed]
- Goslin RH, Skarin AT, Zamcheck N. Carcinoembryonic antigen. A useful monitor of therapy of small cell lung cancer. JAMA 1981;246:2173-6. [Crossref] [PubMed]
- Sawabata N, Maeda H, Yokota S, et al. Postoperative serum carcinoembryonic antigen levels in patients with pathologic stage IA nonsmall cell lung carcinoma: subnormal levels as an indicator of favorable prognosis. Cancer 2004;101:803-9. [Crossref] [PubMed]
- Hu Q, Xiao P, Li J, et al. A retrospective analysis of serum tumor markers found in non-small cell lung cancer. J Cancer Res Ther 2016;12:117-20. [Crossref] [PubMed]
- Lu J, Papp LV, Fang J, et al. Inhibition of Mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Res 2006;66:4410-8. [Crossref] [PubMed]
- Hann SS, Zheng F, Zhao S. Targeting 3-phosphoinositide-dependent protein kinase 1 by N-acetyl-cysteine through activation of peroxisome proliferators activated receptor alpha in human lung cancer cells, the role of p53 and p65. J Exp Clin Cancer Res 2013;32:43. [Crossref] [PubMed]
- Fu L, Chen W, Guo W, et al. Berberine Targets AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and Cytochrome-c/Caspase Signaling to Suppress Human Cancer Cell Growth. PLoS One 2013;8:e69240 [Crossref] [PubMed]
- Xu TP, Shen H, Liu LX, et al. Plumbagin from Plumbago Zeylanica L induces apoptosis in human non-small cell lung cancer cell lines through NF-κB inactivation. Asian Pac J Cancer Prev 2013;14:2325-31. [Crossref] [PubMed]
- Zhang L, Ruan J, Yan L, et al. Xanthatin induces cell cycle arrest at G2/M checkpoint and apoptosis via disrupting NF-κB pathway in A549 non-small-cell lung cancer cells. Molecules 2012;17:3736-50. [Crossref] [PubMed]