Three-year outcomes of 324 prostate carcinoma patients treated with combination high-dose-rate brachytherapy and intensity modulated radiation therapy
Introduction
There are various radiation treatment options for men with prostate cancer. Treatment options include: conventionally fractionated radiation [intensity modulated radiation therapy (IMRT)], stereotactic ablative radiotherapy, and interstitial implantation [high-dose-rate (HDR), low dose rate (LDR)]. Numerous reports have shown a combination of HDR and IMRT confers excellent prostate specific antigen (PSA) control rates (1-3). Some reports have shown improved biochemical control rates for patients treated with combination radiation as compared with patients treated with external beam radiation alone (1,4-10). In this study we report clinical outcomes from combination HDR and IMRT.
Methods
This was a single center study performed at the Cancer Center of Irvine (Irvine, CA). Study candidates included non-metastatic patients with T2–T3b tumors and prostate glands less than 60 cc. We did not classify patients in the cT1 “clinical T1” category as the T1 staging does not accurately quantify the amount of tumor in the prostate. All patients were classified into T2–T3b categories based on biopsy, physical exam, and imaging studies. There were no stage T4 patients. All Gleason and PSA levels were included. Patients were stratified into risk groups according to NCCN Guidelines v1.2011. Gleason 3+4 [7] patients were treated without androgen deprivation therapy (ADT) unless PSA >10. Gleason scores 4+3 [7] and 4+4 [8] were treated with 6 months of ADT. Gleason 9 and 10 received 12 months of ADT. 158 patients (49%) received an absorbable polyethylene glycol (PEG) rectal spacer to help reduce rectal toxicity. All patients consented to having their full clinical data included in our research study.
HDR brachytherapy
Two HDR implants were performed 1 week apart. During each implant procedure, the patient was positioned in the dorsal lithotomy position with either spinal or general anesthesia administered. A Foley catheter was placed into the bladder and expanded with 5 mL of contrast media. A 6.5-megahertz (MHz) endorectal ultrasound probe was inserted to provide ultrasound image guidance, and an interstitial template was secured against the perineum to position and secure the implant needles. Needles were implanted and placed strategically to provide optimal dose conformity. In a typical implant 13 to 15 needles were implanted. Dose was delivered twice daily, once in the morning and once again in the afternoon. Most patients received 4 Gray (Gy) per fraction for a total 8 Gy for each implant, 16 Gy for the two HDR implants combined. The average HDR dose was 17.7 Gy [standard deviation (SD) =3.3 Gy].
HDR plans were generated using the Brachyvision treatment planning program (varian medical systems). The prostate gland was contoured as both the clinical target volume (CTV) and planning target volume (PTV) for treatment planning. The brachytherapy dose was prescribed to the 100% isodose line. Treatment planning goals were as follows: prescribed dose to at least 90% of the CTV (V100 >90), nor more than 40% of the CTV to receive greater than 150% of prescribed dose (V150 <40%), maximum urethral dose under 120%, and no more than 2 cc of either the rectum or bladder to receive greater than 75% of prescribed dose. For 158 patients (49%), a PEG spacer was injected during the second implant to aid in reducing the radiation dose the rectal area. The spacer material resorbs within 6 weeks of implantation.
IMRT
IMRT treatment commenced approximately 1 week after the second HDR implant. A total dose of 59.4 Gy in 33 daily fractions was delivered with IMRT. The mean total IMRT dose was 57.1 Gy (SD =5.4 Gy).
IMRT treatment plans were generated using the Eclipse treatment planning system (varian medical systems). MRI fusion was utilized to better delineate target, normal structures and if applicable, the spacer material. An initial CTV was treated for the first 25 fractions followed by a modified CTV for the final eight fractions. The initial CTV was defined in one of two ways depending on the risk of pelvic lymph node involvement. If the risk of pelvic lymph node involvement was 15% or lower, the initial CTV was defined as the prostate gland and inferomedial 10 mm of the seminal vesicles (11). If the risk of pelvic lymph node involvement was greater than 15%, the initial CTV included the pelvic lymph nodes as defined by Hsu et al. (12). After the first 25 treatments, the CTV was defined as the prostate and inferomedial 10 mm of the seminal vesicles. For both the initial and modified CTV, the CTV was expanded 5–10 mm to produce a PTV. The bladder was contoured as seen on CT, while the rectum was contoured from the ischial tuberosities to the rectosigmoid intersection.
Treatment planning objectives were as follows: prescribed dose to at least 95% of the PTV (V100 >95%), maximum dose less than 110% of prescribed dose (PTVmax <110%). Rectal dose limits were kept within the Radiation Therapy Oncology Group protocol 0415 for IMRT for localized prostate cancer with no more than 15%, 20%, 25%, 35%, and 50% of the rectal volume receiving 60.4, 56.3, 52.3, 48.3, and 40.3 Gy, respectively.
Image-guided radiation therapy
All patients had five gold fiducial markers inserted into the prostate. The fiducial markers were used for daily image guided radiation therapy. Cone beam computed tomography (CBCT) images were obtained prior to every treatment. Using the original treatment planning CT as a reference, the gold fiducials were aligned precisely in three dimensions. This process was repeated prior to every treatment thus ensuring a high level of treatment accuracy.
PEG spacer use
One hundred and fifty eight patients (49%) were treated in combination with a perirectal spacer made from PEG gel. This procedure has been detailed in previous reports (13), but here will be described briefly. A needle is placed under ultrasound guidance into the anterior perirectal space. 10 cc of the spacer material is injected to create an approximately 1.5 cm separation between the rectum and prostate. The spacer material resorbs and is excreted through the kidneys in roughly 6 weeks.
Biochemical control/overall survival
PSA control was evaluated according to the Phoenix definition of biochemical failure (2 ng/mL over absolute nadir) (14). Biochemical control and overall survival were calculated according to the Kaplan-Meier method. The statistical significant threshold was 0.05.
Gastrointestinal (GI)/genitourinary (GU) toxicity
Patients were evaluated at baseline, weekly during the external beam radiation, and every 3 months for the first year. If PSA results were stable the first year, patients were then evaluated every 4–6 months thereafter. Acute toxicity was defined as toxicity occurring during radiation and within 90 days after finishing radiation treatment.
Late toxicity was defined as all adverse events occurring 90 days after finishing treatment. All GI/GU adverse events from radiation treatment were recorded and graded by a board of physicians. GI and genito-urinary symptoms were graded during radiation treatments according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 grading scheme.
Results
Biochemical control/overall survival/distant metastasis
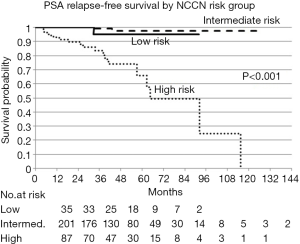
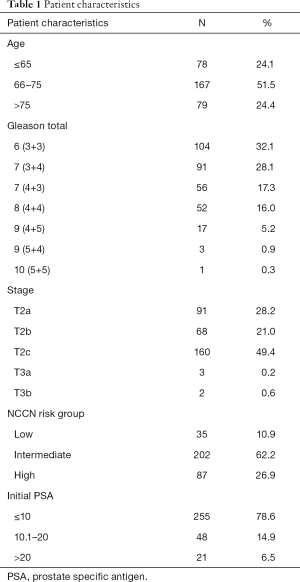
Of the 324 patients treated: 35 were low risk, 201 were intermediate risk, and 87 were high risk. Patient characteristics are presented in Table 1. The average age at diagnosis was 70 years old (SD: ±7). The 3-year PSA relapse free survival rates were 95% [95% confidence interval (CI), 70.7–99.3%], 99% (95% CI, 92.9–99.9%), and 83.4% (95% CI, 71.2–90.7%) for low, intermediate, and high risk patients respectively. PSA relapse free survival by NCCN risk group is presented in Figure 1. Median follow-up for the whole group was 31 months.
Full table
There were no distant metastasis developments in the low or intermediate risk groups. There were five distant metastasis observed in the high risk group. The mean time to development of metastasis was 48 months with a range of 5–128 months.
Five-year overall survival for the whole cohort was 94% (95% CI, 88.0–97.0%). Multivariate analysis showed that age (>65), PSA (>10), and Gleason scores (≥8) were predictive of decreased biochemical control.
Acute GU toxicity
Acute grade 1, 2, and 3 GU toxicities were 36%, 0.4%, and 0%. Most grade 1 toxicities consisted of increase in urinary frequency which on average resolved in 7 months.
Late GU toxicity
Late grade 1, 2, and 3 GU toxicities were 9.0%, 3.0%, and 1.5%. GU toxicities included urethral strictures, urinary retention, hematuria, urinary tract pain, and urinary urgency. Four patients developed grade 3 GU toxicity which consisted of: (I) bladder neck stricture; (II) urethral stricture; (III) hematuria; and (IV) urinary tract pain. Average time to development of grade 3 late toxicity was 26 months (range, 18–33 months). Three of the 4 patients with grade 3 late GU toxicity had a history of previous transurethral resection of prostate (TURP).
Acute GI toxicity
Twenty three percent of patients experience grade 1 toxicity during the radiation treatment which consisted mainly of mild diarrhea. Three patients (1%) developed grade 2 toxicity which represented moderate diarrhea. Average time to resolution of acute GI symptoms was 6 months. No patients developed an acute grade 3 or 4 reaction.
Late GI toxicity
The 3 years incidence of late grade 1, 2 and 3 rectal toxicities were 6%, 2.2%, and 0.4%. All late grade 1 and 2 reactions eventually resolved symptomatically within 1 year after treatment. The patient with a late grade 3 reaction developed severe proctitis 17 months after finishing radiation. The patient developed a mixture of severe fecal obstruction, explosive diarrhea, and fecal incontinence. He was treated with a combination of laxatives, stool softeners, and hydrocortisone suppositories. His symptoms resolved after 9 months and did not require any surgical procedures.
Discussion
Several studies have highlighted the importance of dose escalation in the treatment of intermediate and high risk prostate cancer (2-3,15). With dose escalation, however it is predicted that men will experience more acute and chronic toxicities. Our data shows that with our HDR brachytherapy/IMRT combination, PSA control rates are extremely high with very low early and late side effects. Despite limited 3-year of follow-up, our results are consistent with data reported by other institutions that practice combined modality radiation for the treatment of prostate cancer (1,5,15). Three-year biochemical control rates were excellent for the low and intermediate risk patients at 95% and 97% respectively. It is interesting to note that biochemical control was higher for the intermediate risk patients than the low risk patients. We attribute this to statistical variation resulting from the low number of patients in the low risk arm. There were no biochemical failures in patients with Gleason 3+4 [7] prostate cancer, showing that ADT is not critical in their treatment management. The clinical outcomes of our patients receiving combined modality radiation therapy for prostate cancer are consistent with other comparison studies (1) which have shown improved PSA relapse free survival with the addition of HDR brachytherapy to external beam radiation. Deutsch (1) showed improved 5-year actuarial PSA relapse-free survival (PRFS) for combination therapy (HDR/IMRT) vs. ultra-high-dose IMRT: 100% vs. 98% (P=0.71), 98% vs. 84% (P<0.001), and 93% vs. 71% (P=0.23), for NCCN low, intermediate, and high-risk groups, respectively.
Almost half (49%) of patients received a PEG spacer along with radiation. The spacer does not appear to have an appreciable effect on the biochemical control rates of our patients, as our control rates are very similar to other published series (1-5). GI toxicity was very low with only one grade 3 rectal complication that resolved without surgical intervention.
Overall our treatment fractionation has much lower rates of acute and late GU and GI side effects as compared with other published series (16). This is likely because our HDR fractionation consists of smaller more frequent doses. Since the doses per fraction we use are lower than other centers, it makes radiobiological sense that the toxicity profile is much lower. Despite the protracted HDR fractionation, biochemical control does not appear to be compromised but leads to a decrease in acute and late GI/GU toxicities.
Another limitation of this study was that most high risk patients only received 12 months of hormonal therapy instead of 2–3 years of hormonal blockade as recommended by NCCN guidelines. A longer course of hormonal therapy could possibly lead to improved biochemical control in high risk patients.
Conclusions
We continue to show that the combination HDR/IMRT therapy results in high PSA control rates with minimal GI and GU toxicities.
Acknowledgments
Funding: A portion of this study was funded by a research grant from the Medici Foundation (Irvine, CA, USA).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.85). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval was waived. All patients consented to having their full clinical data included in our research study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deutsch I, Zelefsky MJ, Zhang Z, et al. Comparison of PSA relapse-free survival in patients treated with ultra-high-dose IMRT versus combination HDR brachytherapy and IMRT. Brachytherapy 2010;9:313-8. [Crossref] [PubMed]
- Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002;53:1097-105. [Crossref] [PubMed]
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol 2010;28:1106-11. [Crossref] [PubMed]
- Hsu IC, Cabrera AR, Weinberg V, et al. Combined modality treatment with high-dose-rate brachytherapy boost for locally advanced prostate cancer. Brachytherapy 2005;4:202-6. [Crossref] [PubMed]
- Demanes DJ, Rodriguez RR, Schour L, et al. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10-year results. Int J Radiat Oncol Biol Phys 2005;61:1306-16. [Crossref] [PubMed]
- Chin YS, Bullard J, Bryant L, et al. High dose rate iridium-192 brachytherapy as a component of radical radiotherapy for the treatment of localised prostate cancer. Clin Oncol (R Coll Radiol) 2006;18:474-9. [Crossref] [PubMed]
- Yamada Y, Bhatia S, Zaider M, et al. Favorable clinical outcomes of three-dimensional computer-optimized high-dose-rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy 2006;5:157-64. [Crossref] [PubMed]
- Ares C, Popowski Y, Pampallona S, et al. Hypofractionated boost with high-dose-rate brachytherapy and open magnetic resonance imaging-guided implants for locally aggressive prostate cancer: a sequential dose-escalation pilot study. Int J Radiat Oncol Biol Phys 2009;75:656-63. [Crossref] [PubMed]
- Knybel L, Cvek J, Molenda L, et al. Analysis of Lung Tumor Motion in a Large Sample: Patterns and Factors Influencing Precise Delineation of Internal Target Volume. Int J Radiat Oncol Biol Phys 2016;96:751-8. [Crossref] [PubMed]
- Kotecha R, Yamada Y, Pei X, et al. Clinical outcomes of high-dose-rate brachytherapy and external beam radiotherapy in the management of clinically localized prostate cancer. Brachytherapy 2013;12:44-9. [Crossref] [PubMed]
- Roberts T, Roach M 3rd. The evolving role of pelvic radiation therapy. Semin Radiat Oncol 2003;13:109-20. [Crossref] [PubMed]
- Hsu A, Pawlicki T, Luxton G, et al. A study of image-guided intensity-modulated radiotherapy with fiducials for localized prostate cancer including pelvic lymph nodes. Int J Radiat Oncol Biol Phys 2007;68:898-902. [Crossref] [PubMed]
- Yeh J, Lehrich B, Tran C, et al. Polyethylene glycol hydrogel rectal spacer implantation in patients with prostate cancer undergoing combination high-dose-rate brachytherapy and external beam radiotherapy. Brachytherapy 2016;15:283-7. [Crossref] [PubMed]
- Thames H, Kuban D, Levy L, et al. Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys 2003;57:929-43. [Crossref] [PubMed]
- Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol 2011;60:1133-9. [Crossref] [PubMed]
- Mohammed N, Kestin L, Ghilezan M, et al. Comparison of acute and late toxicities for three modern high-dose radiation treatment techniques for localized prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:204-12. [Crossref] [PubMed]