Downregulation of ZFX is associated with inhibition of prostate cancer progression by baicalein
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed lethal tumors around the world (1). For decades, androgen-deprivation therapy (ADT) has been the standard treatment for advanced PCa (2). However, ADT becomes ineffective no more than 2 years for most PCa progressing into castration-resistant prostate cancer (CRPC) stage, which results in high mortality due to lack of cheap and effective alternative (3,4). Therefore, the development of pharmacological interventions is vital.
Baicalein is a bioactive flavonoid extract obtained from scutellaria baicalensis georgi’s dry root, which are widely used in China. Recently, increasing evidence has suggested that baicalein has anti-tumor effect (5,6). Cellular and animal experiments have verified that baicalein could inhibit tumor cell proliferation, invasiveness and metastasis (7-11) in many cancers, including PCa (12,13). However, the anti-tumor effects and the specific molecular mechanisms in PCa is yet to be further elucidated.
It has been suggested that the inhibition of gallbladder cancer cells by baicalein is correlated with the downregulation of zinc finger protein X-linked (ZFX) (14), which is one kind of zinc-finger protein encoded by ZFX gene (chr Xp21.3). It has also been demonstrated that ZFX plays a vital role in the self-renewal of both embryonic and hematopoietic stem cells (15). Increasing evidence suggests that ZFX acts as an oncogene in many malignant tumors, including glioblastoma (16), hepatocellular (17) and oral squamous carcinoma (18), performing in the regulation of those genes which are essential for cell growth, differentiation and apoptosis. Our previous results also demonstrated that knockdown of ZFX inhibited cell proliferation and induced apoptosis in PC-3 and DU145 cells (19), which indicated that ZFX was critical in PCa cell proliferation and survival. Therefore, we sought to determine whether baicalein could inhibit PCa progression by downregulating ZFX expression.
Methods
Chemicals and antibodies
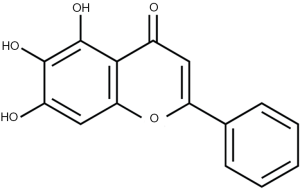
Baicalein was obtained from Sigma-Aldrich (St Louis, Missouri, USA), and its chemical structure is shown in Figure 1. Antibody against ZFX was purchased from Abcam (Cambridge, MA, UK), and antibody against β-actin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Drug treatment
Baicalein [dissolved in dimethyl sulfoxide (DMSO)] was used for experiment group. The final concentration of DMSO (Sigma-Aldrich, St Louis, USA) in each group was 0.1% (v/v). Control cells were treated with vehicle (DMSO group) or blank (CTRL group).
Cell culture
PCa cells of PC-3, DU145 and LNCaP were purchased from Chinese Academy of Sciences (CAS, Shanghai, China). PC-3 and DU145 were cultured in DMEM/F12 medium (Hyclone, USA), and LNCaP was cultured in RPMI-1640 medium (Hyclone, USA) containing 10% FBS. All media were supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin; Gibco, USA). All cells were grown at 37 °C/5% CO2.
CCK-8 assay
Cell proliferation and viability were determined using Cell Counting Kit-8 (CCK-8, Dojindo, Japan) assay. Briefly, cells were seeded at 5×103 cells/well (100 µL) in 96-well plates and cultured overnight. Then different concentrations of baicalein (12.5, 25, 50, 75, 100, 150 and 200 µM) were added. Blank control group (CTRL) and negative control group (vehicle: DMSO) were set up. After treatment for 24, 48, and 72 h, CCK-8 (10 µL, 10%) was added to each well for incubation at 37 °C for 4 h. Then we measured the absorbance at 450 nm with a microplate reader (Molecular Devices, CA, USA). All experiments were repeated in triplicate.
Western blot
Cells were stimulated with different concentrations of baicalein [0, 100 and 200 µmol/L (µM)] for 48 h, then harvested on ice and lysed in RIPA buffer (Beyotime, China) containing 1% protease inhibitors. Proteins were extracted, then separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane for western blotting analyses. The membrane was blocked in 5% non-fat milk in TBST for 2 h. Primary antibodies against ZFX (1:1,000) and β-actin (1:1,000) were incubated at 4 °C overnight. HRP-conjugated IgG were used as secondary antibodies at 1:5,000. The signals were visualized using the enhanced chemiluminescence method.
Immunohistochemistry
Antigen retrieval was conducted in 0.01 mol/L citric acid (pH 6.0). Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide. Primary antibody was diluted 1:100 and incubated overnight at 4 °C, followed by incubation with a biotinylated secondary antibody (1:200) for 1 hour. Immunohistochemical staining was conducted following the avidin-biotin peroxidase complex method with diaminobenzidine as a chromogen. Slides were counterstained with hematoxylin, dehydrated and mounted. Brown cytoplasmic staining of stromal or tumor cells was considered positive.
Wound healing assay
Cells were seeded in 24-well plates and cultured to at least 90% of confluence. Adherent cells were scratched with a plastic 200 µL pipette tip and then washed with phosphate-buffered saline (PBS). Baicalein (25, 50, 100 and 200 µM) was added and cells were cultured at 37 °C/5%CO2. Blank control group (CTRL) and negative control group (vehicle: DMSO) were included. The “wounded” areas were photographed by phase contrast microscopy at 0, 24 and 48 h.
Transwell invasion assay
The cell invasion ability was analyzed by transwell assay as previously described (20). Briefly, cells were pretreated with either 100 µM baicalein or DMSO vehicle control for 24 h, then surviving cells were collected and incubated in transwell chamber (Corning Incorporated, Corning NY, USA) for 24 h. Afterwards, the inserts were taken out from the chamber, and cells on the upper side were carefully removed by swabbing, while cells on the bottom side were fixed, stained and measured.
Establishment and treatment of DU145 xenografts
All animal experiments were approved by the Huashan Hospital Animal Ethics Committee (KY2016-112). Male BABL/c nude mice (The Animal Center of Chinese Academy of Science, Shanghai, China) at the age of 5 weeks were subcutaneously inoculated in the right armpit of each mouse with DU145 cells (5×106 cells/mouse). The mice were housed in a constant room temperature with a 12/12 h light/dark cycle, and ad libitum accessed to food and water. After 2 weeks of tumor formation, mice were randomly divided into two groups (5/group). In these two groups, nude mice bearing DU145 tumor were i.p. injected with 0.1% DMSO and baicalein (30 mg/kg, once/day) for 21 days, respectively. Tumors were measured and tumor weights were detected.
Statistical analysis
The results are presented as mean ± standard deviation (SD). Statistical analysis was performed using t-test by GraphPad Prism. P<0.05 is considered significantly different. Each experiment was repeated at least three times.
Results
Baicalein suppressed the proliferation ability of PCa cells
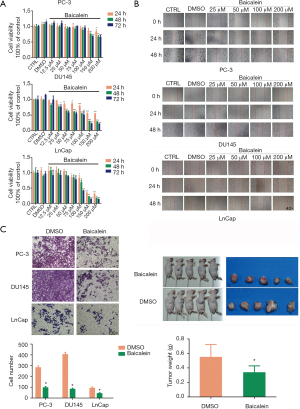
When compared with the control groups (CTRL and DMSO), baicalein significantly inhibited PC-3, DU145 and LNCaP cells proliferation at various concentrations for 24, 48 and 72 h (P<0.05, Figure 2A). And this inhibition effect increased gradually in a time- and dose-dependent manner from 24 to 72 h and from low to high concentration.
Baicalein inhibited the migration and invasion abilities of PCa cells
To analyze the cell metastatic potential, we detected the cell migration capacity used wound healing assay. As shown in Figure 2B, cells in baicalein groups had weaker migration ability than those of control groups (CTRL and DMSO) for 24 and 48 h in PC-3, DU145 and LNCaP cells. And in higher concentration of baicalein groups, the scratch width was wider at 24 and 48 h. These results showed that baicalein inhibited the migration ability of PCa cells in a time- and dose-dependent manner. Transwell assays were performed to assess cell invasion ability. Consistently, the results showed that the number of cells which invaded to the lower chamber in baicalein group was lower than DMSO vehicle control group (Baicalein/DMSO: 33.7%, 20.3%, 46.2% in PC-3, DU145 and LNCaP cells, respectively). Baicalein significantly inhibited the invasion ability of cells compared with DMSO vehicle control (P<0.05, Figure 2C).
Baicalein suppressed the tumor growth of DU145 xenografts
To test the antitumor activity of baicalein in vivo, nude mice bearing human prostate adenocarcinoma (DU145) tumors were treated with baicalein or DMSO (as control) by intraperitoneal injection for 3 weeks. As shown in Figure 2D, baicalein inhibited growth of human prostate adenocarcinoma (DU145) cells and decreased tumor size in appearance when compared with the control group (DMSO). Similarly, the tumor weights were significantly inhibited by baicalein when compared with DMSO (0.336±0.092 vs. 0.548±0.176 g, P<0.05).
Baicalein inhibited the expression of ZFX protein in vitro and in vivo
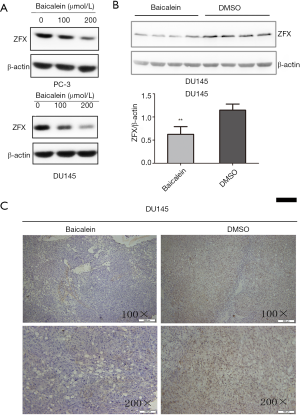
To assess whether baicalein downregulates ZFX protein expression in PCa, the levels of ZFX were detected after baicalein treatment using western blot analysis and immunohistochemistry assay. As expected, baicalein treatment induced a decrease in ZFX protein levels in vitro and in vivo. In vitro, western blot analysis (Figure 3A) showed that ZFX protein levels decreased in a dose-dependent manner with the baicalein administration in PC-3 and DU145 cells. To further study the downregulation effects in vivo, the nude mouse transplantation tumor experiment and immunohistochemistry assay were used. Nude mice bearing human prostate adenocarcinoma (DU145) tumors were administered baicalein or DMSO vehicle control by intraperitoneal injection for 3 weeks. Then we conducted western bolt and immunohistochemistry assay to examine the expression of ZFX protein in tumors from nude mice mentioned above. As shown in Figure 3B,C, the levels of ZFX decreased significantly in the tumors following treatment with baicalein compared with DMSO.
Discussion
In our study, we show that baicalein has a significantly inhibitory effect both in vitro and in vivo on progression of human PCa cells. These findings are similar to prior study (13), except that they conducted their in vivo study on SCID mice by oral administration of baicalein. In our study, we administrated baicalein by i.p. injection because its reabsorption is not easy due to its poor permeability. from the gastrointestinal tract (21). Further, we show that baicalein suppress the expression of ZFX, knockdown of which inhibited PCa cell proliferation and induced apoptosis in our previous study (19).
Patients have limited methods of treatment when progressing into metastatic PCa due to loss of sensitivity to androgen withdrawal. Formerly, PC-SPES had been one kind of prevalent herbal supplements chosen by those patients (22,23). As a mixture of 8 herbs, including baicalein as a main ingredient (24), PC-SPES quickly lowered PSA levels in patients with PCa and also improved quality of life and reduced pain (25). However, because FDA-regulated compounds such as the synthetic nonsteroidal estrogen diethylstilbestrol (DES) were included in PC-SPES (26), it was taken off the market in 2002.
Baicalein was seen as a major component of PC-SPES in inhibiting cancer growth (24). It is an important member of flavonoids family, which have been reported to have anti-allergic, anti-inflammatory and anti-microbial effects (27,28), and have been widely used in China for a long time. In addition, many studies have suggested that flavonoids possess anti-tumor ability against various cancer (29-31), the underlying mechanisms include suppression of cell proliferation, induction of apoptosis and cycle arrest (32). In addition, Gu et al. revealed that baicalein had a potential feature of targeting cancer stem cells of multiple myeloma (33), and Liu et al. showed that baicalein downregulated ZFX expression in gallbladder cancer cells (14).
In non-malignant embryonic stem cell, the transcriptional role of ZFX in the self-renewal of both embryonic and hematopoietic stem cells was described (15,34). Using a new software program, Ouyang et al. identified ZFX as one of 12 transcription factors upregulated in embryonic stem cells (35). And in malignant cells, previous studies have indicated that ZFX can function as an oncoprotein in various types of cancer like gastric cancer (36), gallbladder cancer (37), hepatocellular cancer (17) and PCa (19). Therefore, suppression of ZFX would be an operative therapeutic approach to cancer treatment, including PCa.
Conclusions
We demonstrated that baicalein could significantly inhibit the progression of PCa, and downregulate the expression of ZFX in vitro and in vivo. Due to the important role of ZFX in PCa progression, we have a reasonable speculation that the inhibition is probably associated with the downregulation of ZFX, and we expected that baicalein could play significant role in PCa treatment in the future.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (grant number 81272835) and Outstanding Talents Cultivation Project of Shanghai Medical System (grant number XBR2013092).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.09.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived. The study was approved by the Huashan Hospital Animal Ethics Committee (No. KY2016-112).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Matsumoto K, Tanaka N, Hayakawa N, et al. The type of patients who would benefit from anti-androgen withdrawal therapy: could it be performed safely for aggressive prostate cancer? Med Oncol 2013;30:647. [Crossref] [PubMed]
- Mohler JL, Gregory CW, Ford OH 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res 2004;10:440-8. [Crossref] [PubMed]
- Agoulnik IU, Weigel NL. Androgen receptor action in hormone-dependent and recurrent prostate cancer. J Cell Biochem 2006;99:362-72. [Crossref] [PubMed]
- Hui C, Qi X, Qianyong Z, et al. Flavonoids, flavonoid subclasses and breast cancer risk: a meta-analysis of epidemiologic studies. PLoS One 2013;8:e54318 [Crossref] [PubMed]
- Geybels MS, Verhage BA, Arts IC, et al. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am J Epidemiol 2013;177:1388-98. [Crossref] [PubMed]
- Cheng YH, Li LA, Lin P, et al. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol Appl Pharmacol 2012;263:360-7. [Crossref] [PubMed]
- Ma GZ, Liu CH, Wei B, et al. Baicalein inhibits DMBA/TPA-induced skin tumorigenesis in mice by modulating proliferation, apoptosis, and inflammation. Inflammation 2013;36:457-67. [Crossref] [PubMed]
- Naveenkumar C, Raghunandhakumar S, Asokkumar S, et al. Baicalein abrogates reactive oxygen species (ROS)-mediated mitochondrial dysfunction during experimental pulmonary carcinogenesis in vivo. Basic Clin Pharmacol Toxicol 2013;112:270-81. [Crossref] [PubMed]
- Chen J, Li Z, Chen AY, et al. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int J Mol Sci 2013;14:6012-25. [Crossref] [PubMed]
- Li X, Zou K, Gou J, et al. Effect of baicalin-copper on the induction of apoptosis in human hepatoblastoma cancer HepG2 cells. Med Oncol 2015;32:72. [Crossref] [PubMed]
- Guo Z, Hu X, Xing Z, et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol Cell Biochem 2015;406:111-9. [Crossref] [PubMed]
- Miocinovic R, McCabe NP, Keck RW, et al. In vivo and in vitro effect of baicalein on human prostate cancer cells. Int J Oncol 2005;26:241-6. [PubMed]
- Liu TY, Gong W, Tan ZJ, et al. Baicalein inhibits progression of gallbladder cancer cells by downregulating ZFX. PLoS One 2015;10:e0114851 [Crossref] [PubMed]
- Galan-Caridad JM, Harel S, Arenzana TL, et al. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell 2007;129:345-57. [Crossref] [PubMed]
- Fang X, Huang Z, Zhou W, et al. The zinc finger transcription factor ZFX is required for maintaining the tumorigenic potential of glioblastoma stem cells. Stem Cells 2014;32:2033-47. [Crossref] [PubMed]
- Lai KP, Chen J, He M, et al. Overexpression of ZFX confers self-renewal and chemoresistance properties in hepatocellular carcinoma. Int J Cancer 2014;135:1790-9. [Crossref] [PubMed]
- Ma H, Yang F, Lian M, et al. Dysregulation of zinc finger protein, X-linked (ZFX) impairs cell proliferation and induces apoptosis in human oral squamous cell carcinorma. Tumour Biol 2015;36:6103-12. [Crossref] [PubMed]
- Jiang H, Zhang L, Liu J, et al. Knockdown of zinc finger protein X-linked inhibits prostate cancer cell proliferation and induces apoptosis by activating caspase-3 and caspase-9. Cancer Gene Ther 2012;19:684-9. [Crossref] [PubMed]
- Liu B, Wang G, Yang J, et al. Berberine inhibits human hepatoma cell invasion without cytotoxicity in healthy hepatocytes. PLoS One 2011;6:e21416 [Crossref] [PubMed]
- Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos 2002;30:370-7. [Crossref] [PubMed]
- Hsieh TC, Lu XH, Chea J, et al. Prevention and management of prostate cancer using PC-SPES: A scientific perspective. J Nutr 2002;132:3513S-7S. [PubMed]
- Cao H, Mu Y, Li X, et al. A Systematic Review of Randomized Controlled Trials on Oral Chinese Herbal Medicine for Prostate Cancer. PLoS One 2016;11:e0160253 [Crossref] [PubMed]
- Ikezoe T, Chen SS, Heber D, et al. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate 2001;49:285-92. [Crossref] [PubMed]
- Pfeifer BL, Pirani JF, Hamann SR, et al. PC-SPES, a dietary supplement for the treatment of hormone-refractory prostate cancer. BJU Int 2000;85:481-5. [Crossref] [PubMed]
- Guns ES, Goldenberg SL, Brown PN. Mass spectral analysis of PC-SPES confirms the presence of diethylstilbestrol. Can J Urol 2002;9:1684-8; discussion 9.
- Wall C, Lim R, Poljak M, et al. Dietary Flavonoids as Therapeutics for Preterm Birth: Luteolin and Kaempferol Suppress Inflammation in Human Gestational Tissues In Vitro. Oxid Med Cell Longev 2013;2013:485201
- Mahboubi M, Kazempour N, Boland Nazar AR. Total phenolic, total flavonoids, antioxidant and antimicrobial activities of scrophularia striata boiss extracts. Jundishapur J Nat Pharm Prod 2013;8:15-9. [Crossref] [PubMed]
- Chiu YW, Lin TH, Huang WS, et al. Baicalein inhibits the migration and invasive properties of human hepatoma cells. Toxicol Appl Pharmacol 2011;255:316-26. [Crossref] [PubMed]
- Wu JY, Tsai KW, Li YZ, et al. Anti-Bladder-Tumor Effect of Baicalein from Scutellaria baicalensis Georgi and Its Application In Vivo. Evid Based Complement Alternat Med 2013;2013:579751
- Wang L, Ling Y, Chen Y, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett 2010;297:42-8. [Crossref] [PubMed]
- Liang RR, Zhang S, Qi JA, et al. Preferential inhibition of hepatocellular carcinoma by the flavonoid Baicalein through blocking MEK-ERK signaling. Int J Oncol 2012;41:969-78. [Crossref] [PubMed]
- Gu YY, Liu LP, Qin J, et al. Baicalein decreases side population proportion via inhibition of ABCG2 in multiple myeloma cell line RPMI 8226 in vitro. Fitoterapia 2014;94:21-8. [Crossref] [PubMed]
- Cellot S, Sauvageau G. Zfx: at the crossroads of survival and self-renewal. Cell 2007;129:239-41. [Crossref] [PubMed]
- Ouyang Z, Zhou Q, Wong WH. ChIP-Seq of transcription factors predicts absolute and differential gene expression in embryonic stem cells. Proc Natl Acad Sci U S A 2009;106:21521-6. [Crossref] [PubMed]
- Wu S, Lao XY, Sun TT, et al. Knockdown of ZFX inhibits gastric cancer cell growth in vitro and in vivo via downregulating the ERK-MAPK pathway. Cancer Lett 2013;337:293-300. [Crossref] [PubMed]
- Weng H, Wang X, Li M, et al. Zinc finger X-chromosomal protein (ZFX) is a significant prognostic indicator and promotes cellular malignant potential in gallbladder cancer. Cancer Biol Ther 2015;16:1462-70. [Crossref] [PubMed]