
A tiny but crucial player bridging microbes and colonic carcinogenesis
Colorectal cancer (CRC) is one of the most common malignancies in the world (1). The probability of suffering from CRC is approximately 4–5%, and the risk of developing CRC is associated with characteristics such as age, chronic disease history, and lifestyle (2). CRC is caused by mutations in oncogenes, tumor-suppressor genes, and genes related to DNA repair mechanisms. The underlying pathogenic mechanisms are threefold; namely, chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP). In CRC, common mutations, chromosomal changes, and translocations reportedly affect important intracellular signaling pathways [WNT, mitogen activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K), transforming growth factor (TGF)-β, and TP53] (2). In addition to genetic mutations, alterations in non-coding RNAs, such as microRNAs, also contribute to carcinogenesis.
The intestinal microbiota is involved in the pathogenesis of intestinal diseases (3). Fusobacterium nucleatum is a commensal oral microorganism associated with gingivitis and periodontitis. However, this microbe is also involved in non-oral diseases, such as colorectal and pancreatic cancers (4,5). Metagenomics studies have reported enrichment of F. nucleatum in human CRC and adenoma tissues, compared with adjacent tissues (6,7). Subsequent in vitro and in vivo studies showed that F. nucleatum accelerates the progression of CRC, suggesting that it induces tumor growth and enhances tumor survival (5,8-10).
MicroRNAs are a family of small (19–22 nucleotides), non-coding RNAs that post-transcriptionally regulate gene expression. Approximately 30% of human genes are regulated by microRNAs. This regulation has implications for numerous important cellular functions. Given the critical regulatory roles of microRNAs, it is unsurprising that they are associated with carcinogenesis. Many microRNAs, including microRNA-21, which mediates cell growth and tumor progression, are upregulated in CRC. Human microRNA-21 (hsa-miR-21) is located on chromosome 17q23-1 overlapping with the TMEM49 gene, a human homologue of rat vacuole membrane protein-1. MicroRNA-21 is regulated by its own promoter, which contains binding sites for the transcription factors AP-1 and PU.1 (11). MicroRNA-21 functions in many cell types as an anti-apoptotic and pro-survival factor, and plays an important role in cancer biology, including in adenoma and CRC (12-15).
The study by Yang et al. showed that F. nucleatum induces proliferation of colon tumor cells by activating Toll-like receptor 4 (TLR4) and the nuclear factor (NF)-κB pathway, leading to increased levels of microRNA-21 (16). Using an Apcmin/+ model, they determined that F. nucleatum stimulates the immune response and promotes tumorigenesis due to mutations in the APC gene. Several miRNAs, including microRNA-21, were upregulated by F. nucleatum and microRNA-21 was shown to be a direct target of F. nucleatum. That is, F. nucleatum upregulates microRNA-21 expression by activating TLR4 and NF-κB. Using microRNA-21—knockout (miR21a–/–) mice, the authors evaluated the role of microRNA-21 upregulation in colon tumor cell proliferation. In addition, RASA1, a suppressor of Ras, was identified as a novel and direct target of microRNA-21, which, in turn, activates the MAPK pathway, leading to cell proliferation. These results suggest that F. nucleatum plays a role as a driver, not simply a passenger, in colorectal tumorigenesis, by upregulating the expression of microRNA-21 (Figure 1). This study provides solid evidence for an association between F. nucleatum and CRC, and suggests that F. nucleatum induces colorectal tumorigenesis in a manner dependent on microRNA-21.
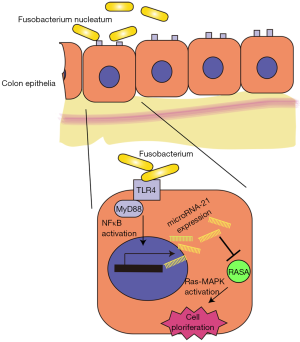
These novel findings provide interesting insight into the role of F. nucleatum in colonic carcinogenesis, a role that involves upregulation of microRNA-21. These exciting results shed new light on this field, but also suggest further important questions.
The strain diversity of F. nucleatum should be taken into consideration in any future works. Similarly, F. nucleatum exists in a complex community of other microorganisms. Thus, it may be necessary to investigate the role of the intestinal microbiota in F. nucleatum-induced colonic carcinogenesis. Such analyses may provide information on why F. nucleatum induces CRC at a specific stage of life or in specific cases. In addition, while this may also be related to strain diversity, the F. nucleatum virulence factors involved in colonic carcinogenesis should be determined. Fusobacterial lectin Fap2 reportedly interacts with host Gal-GalNac (17), and fusobacterial FadA adheres to host cadherins, which leads to activation of oncogenic beta-catenin signaling (10). These results, together with the findings of Yang and colleagues (16), suggest that F. nucleatum interferes with host cellular homeostasis via multiple pathways.
Another important question regarding F. nucleatum-related colonic carcinogenesis is the global prevalence of this agent. There are significant differences in the prevalence of F. nucleatum in CRCs among countries. For example, 13% of colon carcinoma tissues were F. nucleatum positive in a study in the United States (4), while only 8.6% were positive in Japanese cases (4). Thus, the causative role of this pathogen in colonic carcinogenesis should take into consideration the geographical differences. Other microbes may also play crucial roles in colonic carcinogenesis in some countries. Thus, the significance in colonic carcinogenesis and distribution of F. nucleatum should be clarified further. In addition, because microRNA-21 is overexpressed in most solid tumors, it is possible that microbes other than F. nucleatum induce microRNA-21 expression.
MicroRNA-21 is commonly overexpressed in solid tumors, such as those of the lung, breast, stomach, prostate, colon, brain, head and neck, esophagus, and pancreas (15). MicroRNA-21 reportedly targets PDCD4, CdC25A, TGF-β R2, or other genes, some of which may be involved in colonic carcinogenesis. The study by Yang et al. suggested RASA as a novel target of microRNA-21, which promotes CRC cell proliferation. However, RASA is likely not the only gene responsible for the induction of microRNA-21-mediated colonic carcinogenesis.
Regulation of microRNA expression is a hot topic. c-fos and the AP-1 family are involved in miroRNA-21 promoter activity (11). Yang et al. found that NF-κB influenced microRNA-21 promoter activity, which is consistent with a previous report (18). However, microRNA-21 also targets Pelino, which negatively regulates NF-κB activity (19). Thus, the mechanism by which F. nucleatum activates the microRNA-21 promoter, and whether this is provoked solely by activation of the NF-κB pathway, should be subjects of future works.
CRCs can be classified as sporadic, inflammation-associated, or inherited. The contribution of F. nucleatum to colorectal carcinogenesis in general is unclear. For example, colitis-associated cancer may be different pathologically from sporadic or inherited cancers. Indeed, although microRNA expression levels are unchanged, microRNA function is globally deregulated in the presence of chronic inflammation (20), which is closely associated with colitis-associated carcinogenesis (21). Because Yang et al. used dextran sulfate sodium (DSS)-induced colitis-associated colon cancer model to evaluate the role of F. nucleatum, it will be interesting to assess microRNA function in F. nucleatum-infected lesions.
A crucial question regarding F. nucleatum and colonic carcinogenesis is whether this pathogen induces the genetic abnormalities detected in most CRCs. There is some speculation about an indirect linkage between F. nucleatum and MSI due to reduced activity of mismatch repair mechanisms caused by reactive oxygen species (ROS) produced as a result of infection (8). However, no direct linkage between F. nucleatum infection and induction of genetic mutations has been reported to date. Yang et al. used in vivo Apcmin/+ and azoxymethane (AOM)/DSS models, which may involve genetic mutations in Apc or random mutations caused by AOM in the absence of F. nucleatum infection. Then, strictly speaking, F. nucleatum may not be a pure driver of carcinogenesis, but accelerates the proliferation of colon cancer cells by inducing microRNA-21 expression. Therefore, a relationship between the F. nucleatum level and/or microRNA-21 expression in carcinoma tissues and prognosis is feasible, and these may be clinically useful biomarkers.
As described above, many issues remain to be determined to gain a full understanding of the biological role of F. nucleatum in colon cancers. However, the findings of Yang et al. will facilitate the development of novel therapeutics. Whether eradication of F. ucleatum or inhibition of microRNA-21 function improves the prognosis of a subset of colon cancer patients should be determined. Also, the involvement of microRNAs in the roles of pathogens in pathological conditions should be investigated not only in colon cancers but also in other diseases. It is possible that, similar to F. nucleatum, as-yet-unknown agents regulate microRNA expression in pathological conditions. Efforts to discover such factors will contribute to improvement of human health. In this context, the association between microbes and microRNAs in human diseases is a hot topic that warrants vigorous research.
Acknowledgments
Funding: This work was supported by the Research Program on Hepatitis and the Project for Cancer Research and Therapeutic Evolution (P-CREATE) from the Japan Agency for Medical Research and Development (AMED) (to M.O.).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fei Pan (Department of Gastroenterology and Hepatology, Division of Internal Medicine, PLA Medical School & PLA General Hospital, Beijing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.11.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, et al. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci 2017;18:E197 [Crossref] [PubMed]
- Sommer F, Anderson JM, Bharti R, et al. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol 2017;15:630-8. [Crossref] [PubMed]
- Nosho K, Sukawa Y, Adachi Y, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol 2016;22:557-66. [Crossref] [PubMed]
- Gholizadeh P, Eslami H, Kafil HS. Carcinogenesis mechanisms of Fusobacterium nucleatum. Biomed Pharmacother 2017;89:918-25. [Crossref] [PubMed]
- Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299-306. [Crossref] [PubMed]
- Ray K. Colorectal cancer: Fusobacterium nucleatum found in colon cancer tissue--could an infection cause colorectal cancer? Nat Rev Gastroenterol Hepatol 2011;8:662. [Crossref] [PubMed]
- Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res 2014;74:1311-8. [Crossref] [PubMed]
- Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013;14:207-15. [Crossref] [PubMed]
- Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013;14:195-206. [Crossref] [PubMed]
- Fujita S, Ito T, Mizutani T, et al. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol 2008;378:492-504. [Crossref] [PubMed]
- Shi C, Yang Y, Xia Y, et al. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut 2016;65:1470-81. [Crossref] [PubMed]
- Wang P, Zou F, Zhang X, et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res 2009;69:8157-65. [Crossref] [PubMed]
- Lu Z, Liu M, Stribinskis V, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 2008;27:4373-9. [Crossref] [PubMed]
- Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 2006;103:2257-61. [Crossref] [PubMed]
- Yang Y, Weng W, Peng J, et al. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology 2017;152:851-66.e24. [Crossref] [PubMed]
- Abed J, Emgård JE, Zamir G, et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016;20:215-25. [Crossref] [PubMed]
- Yang CH, Li K, Pfeffer SR, et al. The Type I IFN-Induced miRNA, miR-21. Pharmaceuticals (Basel) 2015;8:836-47. [Crossref] [PubMed]
- Marquez RT, Wendlandt E, Galle CS, et al. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-kappaB signaling. Am J Physiol Gastrointest Liver Physiol 2010;298:G535-41. [Crossref] [PubMed]
- Yoshikawa T, Wu J, Otsuka M, et al. ROCK inhibition enhances microRNA function by promoting deadenylation of targeted mRNAs via increasing PAIP2 expression. Nucleic Acids Res 2015;43:7577-89. [Crossref] [PubMed]
- Yoshikawa T, Wu J, Otsuka M, et al. Repression of MicroRNA Function Mediates Inflammation-associated Colon Tumorigenesis. Gastroenterology 2017;152:631-43. [Crossref] [PubMed]


