
LATITUDE and other coordinates in quality of life of prostate cancer patients
For prostate cancer as for many other solid tumors, cancer treatments are constantly and rapidly evolving. The primary goals remain, however, the same: to allow patients to live longer and to live better (1). Clinical benefit integrates, in fact, treatment effectiveness as well as assessment of quality of life (QoL). Both the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) formally include health-related QoL (HRQoL) results among the parameters considered for the evaluation of clinical value of anticancer treatments (2,3). As a matter of fact, not only cancer itself but also treatments can produce distressing symptoms and serious toxicities, affecting functional domains and QoL (4). Monitoring symptoms and QoL allows a complete definition of benefits and harms associated with treatments. Patient-reported outcomes (PROs) are specifically conceived to describe patient’s own experience about disease symptoms, treatment tolerability and toxicity. These patient-based data correlate with care effectiveness, care outcomes and care satisfaction. Measurement of PROs and patient experience can be useful to improve care and to guide treatment choice, when different effective options are available for the same indication (5).
The rapidly changing therapeutic scenario of metastatic hormone-sensitive prostate cancer
The landscape of metastatic hormone-sensitive prostate cancer (mCSPC) is a good example of the recent evolution of systemic treatments. Namely, two different drugs, already used in patients with castration-resistant prostate cancer, have demonstrated an improvement in overall survival (OS) when added to androgen deprivation therapy (ADT): the cytotoxic drug docetaxel and the new-generation hormonal treatment abiraterone acetate (in combination with prednisone). CHAARTED (6), STAMPEDE (7,8) and LATITUDE (9) are the randomized trials that have changed the previous therapeutic paradigm and are going to change clinical practice.
The CHAARTED trial and the first comparison of the multi-arm multi-stage STAMPEDE study, both of which evaluated ADT alone versus ADT plus 6 cycles of docetaxel in newly diagnosed advanced prostate cancer, demonstrated an OS improvement with this early administration of chemotherapy (6,7) that represents, today, the standard treatment for men with a good performance status allowing cytotoxic treatment.
Two years later, another comparison of the STAMPEDE trial and the LATITUDE study showed the results of the addition of concurrent abiraterone to ADT for the treatment of advanced prostate cancer in a clinically and prognostically similar subset of patients. Namely, in the STAMPEDE trial, men with newly diagnosed metastatic, node-positive, or high-risk locally advanced prostate cancer were randomized, in open-label, to ADT alone versus ADT plus abiraterone and prednisolone. Primary outcomes of the trial were OS and failure-free survival (FFS). Namely, failure was defined as prostate-specific antigen (PSA) failure, local or lymph node failure, distant metastases or prostate cancer-death. After a median follow-up of 40 months, addition of abiraterone and prednisolone was associated with a 37% reduction in the risk of death [hazard ratio, 0.63; 95% confidence interval (CI), 0.52–0.76], a 71% improvement in FFS (hazard ratio, 0.29; 95% CI, 0.25–0.34) and decreased symptomatic skeletal-related events (hazard ratio, 0.46; 95% CI, 0.37–0.58) (8).
In the LATITUDE trial, 1,199 men with newly diagnosed high-risk (characterized by at least two factors among Gleason ≥8 disease, three or more radiographic bone lesions, or presence of visceral metastases) metastatic prostate cancer were randomized to ADT plus abiraterone acetate with prednisone versus ADT plus placebos. The co-primary endpoints of the trial were OS and radiographic progression-free survival (rPFS). With all the limitations of indirect comparisons, efficacy results were very similar to those observed with abiraterone acetate in the STAMPEDE trial, and to results obtained with docetaxel. In detail, after a median follow-up of 30.4 months, patients treated in the experimental arm experienced a 38% reduction in the risk of death (hazard ratio, 0.62; 95% CI, 0.51–0.76). Median OS was 34.7 months in the control arm receiving ADT alone, whereas in the experimental arm median OS was not yet reached. Furthermore, there was a 53% reduction in the risk of radiographic progression or death for patients receiving abiraterone, with a median rPFS equal to 33.0 months, compared to 14.8 months in the control arm (hazard ratio, 0.47; 95% CI, 0.39–0.55) (9).
QoL: looking at results
QoL was an exploratory endpoint of the LATITUDE trial, and QoL results have been recently reported by Chi and colleagues (10) (while report of QoL data from the STAMPEDE trial is still pending). In the LATITUDE trial, PROs were collected using electronic tablet devices, with a pre-fixed frequency. Patients were assessed by several instruments, including Brief Pain Inventory-Short Form (BPI-SF), Brief Fatigue Inventory (BFI), Functional Assessment of Cancer Therapy Prostate scale (FACT-P), and the EuroQol (EQ-5D-5L) questionnaires.
The analysis of BPI-SF and BFI allowed the evaluation of the delay in pain/fatigue progression and pain/fatigue interference. Interestingly, results showed an advantage for the addition of abiraterone to ADT in terms of worst pain progression (37% risk reduction; hazard ratio, 0.63; 95% CI, 0.52–0.77; P<0.0001) and pain interference progression (33% risk reduction; hazard ratio, 0.67; 95% CI, 0.56–0.80; P<0.0001). In addition, the use of abiraterone was associated with a significant improvement in the progression of fatigue (35% risk reduction; hazard ratio, 0.65; 95% CI, 0.53–0.81; P=0.0001) and in the progression of fatigue interference (41% risk reduction; hazard ratio, 0.59; 95% CI, 0.47–0.75; P<0.0001). Furthermore, according to repeated-measures mixed-effects model analysis, mean change from baseline was improved in the ADT plus abiraterone group, for both pain and fatigue, early from the start of treatment (cycles 2–5) and maintained through the following cycles.
The FACT-P scales, including the general FACT (FACT-G) subscale, were used to measure general QoL and prostate-cancer-specific QoL. Median time to deterioration of functional status was 12.9 months for patients assigned to ADT plus abiraterone, versus 8.3 months for patients assigned to ADT plus placebos (hazard ratio, 0.85; 95% CI, 0.74–0.99; P=0.032). In repeated-measures analyses, the FACT-P total and subscales scores at most timepoints, compared with baseline, were similar or better for patients assigned to the experimental arm, than for patients in the control group.
Health status and health utility scores, measured by EQ-5D-5L (11), were also statistically significantly improved in the experimental arm.
In synthesis, clinical benefit for patients treated with abiraterone in LATITUDE trial has been confirmed with both methods used to analyze HRQoL: time to PRO deterioration and linear mixed model for repeated measures. Time to deterioration provides a longitudinal analysis on HRQoL, integrating the information obtained with the analysis of rPFS. The QoL benefit demonstrates that the significant improvement in instrumental control of the disease is associated with a clinically relevant improvement in the control of symptoms. On the other hand, the repeated-measures analyses based on measures made on the same patient at different time points, are useful to estimate the effect even in an early phase, both in terms of symptom control and treatment toxicities.
The QoL results of the LATITUDE trial, showing the significantly longer time to PRO deterioration and the early improving of symptoms, add useful information to the primary efficacy analysis of the study.
However, some critical issues must be highlighted: as in most clinical trials, patients were included on the basis of specific eligibility criteria, had a good performance status (ECOG 0–2) and the consequent results might be not generalizable to the entire population. Furthermore, no imputations for missing PRO data were performed, although rates of compliance were high.
Beyond these limitations, these QoL results can be only indirectly compared to those reported in the CHAARTED trial with the addition of docetaxel to ADT (Table 1). In CHAARTED, conducted in a similar subset of patients, QoL showed a different trend. It was assessed by FACT-P, FACT-Taxane, Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), and BPI at baseline and at 3, 6, 9, and 12 months. Patients in the experimental arm receiving docetaxel reported a decline in scores after 3 months (P=0.003), but differences between baseline and 12 months were not significant. QoL was worse at 3 months in the ADT plus docetaxel group compared with ADT alone (P=0.02), reflecting the toxicity of chemotherapy compared to hormonal treatment alone; on the contrary, after 12 months, scores were significantly better in the docetaxel arm (P=0.04). Of note, however, mean differences were not necessarily clinically relevant, not exceeding the threshold of minimum clinically important difference at any time point. At all time points after baseline, treatment burden, measured by the FACT-Taxane, showed significantly poorer scores for men receiving ADT plus docetaxel than for men receiving ADT alone. Fatigue was significantly greater for patients receiving docetaxel only at 3 months, while pain intensity and pain interference were similar between the two arms at all time points (12).
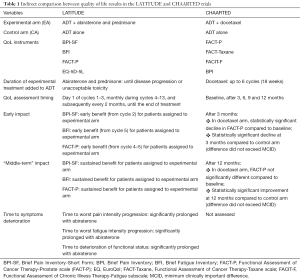
Full table
In synthesis, the improvement in overall QoL at 12 months for patients assigned to ADT plus docetaxel suggests that the early physical and functional negative impact of chemotherapy is reversible, and that the addition of docetaxel in these patients is associated with a possible long-term benefit.
QoL: looking at the future
Considering the results obtained with both the addition of docetaxel and the addition of abiraterone to ADT in patients with metastatic hormone sensitive disease, the question of which agent has to be chosen upfront remains unsolved. Indirect comparisons and network meta-analyses have not demonstrated significant differences in efficacy between the two approaches (13,14). Of note, even the direct comparison between the two strategies, allowed by the contemporaneous randomization within the multi-arm STAMPEDE trial, has not shown a significant difference in OS or in prostate-cancer specific survival, nor in other important outcomes, although this comparison was not the primary endpoint of the trial and was not formally powered (15). Given the similar OS results, QoL, toxicities and cost-effectiveness play a critical role (16). Therefore, in the clinical choice between the two treatments, it is important to consider the markedly different profile of the two drugs. On one hand, six cycles of docetaxel are associated with higher toxicity during the months of treatment, but a long-term benefit; on the other hand, the addition of abiraterone acetate provides a better early outcome in terms of tolerability, but implies a continuous, longer duration of therapy (with both economic implications in terms of treatment cost and clinical implications in terms of adverse events, including the toxicity associated with the chronic steroid treatment).
These considerations underline how important is understanding QOL impact of anticancer therapies (5). Prostate cancer is a disease characterized by relevant heterogeneity in patients’ characteristics, with a high proportion of elderly subjects who suffer from competing risks of disease and disability. PROs and QoL analyses should play a crucial role for a complete decision-making process (17).
The PRO results from the LATITUDE trial can be viewed as an important step in this direction and a tool to guide clinicians in the choice of the best treatment for every single patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hong-Chao He (Department of Urology, Shanghai Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.05.29). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Booth CM, Tannock I. Reflections on medical oncology: 25 years of clinical trials--where have we come and where are we going? J Clin Oncol 2008;26:6-8. [Crossref] [PubMed]
- Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J Clin Oncol 2015;33:2563-77. [Crossref] [PubMed]
- Cherny NI, Dafni U, Bogaerts J, et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol 2017;28:2340-66. [Crossref] [PubMed]
- Esther Kim JE, Dodd MJ, Aouizerat BE, et al. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage 2009;37:715-36. [Crossref] [PubMed]
- Di Maio M, Perrone F. Lessons from clinical trials on quality-of- life assessment in ovarian cancer trials. Ann Oncol 2016;27:961-2. [Crossref] [PubMed]
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737-46. [Crossref] [PubMed]
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomized controlled trial. Lancet 2016;387:1163-77. [Crossref] [PubMed]
- James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338-51. [Crossref] [PubMed]
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352-60. [Crossref] [PubMed]
- Chi KN, Protheroe A, Rodríguez-Antolín A, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol 2018;19:194-206. [Crossref] [PubMed]
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. [Crossref] [PubMed]
- Morgans AK, Chen YH, Sweeney CJ, et al. Quality of Life During Treatment with Chemohormonal Therapy: Analysis of E3805 Chemohormonal Androgen Ablation Randomized Trial in Prostate Cancer. J Clin Oncol 2018;36:1088-95. [Crossref] [PubMed]
- Wallis CJD, Klaassen Z, Bhindi B, et al. Comparison of Abiraterone Acetate and Docetaxel with Androgen Deprivation Therapy in High-risk and Metastatic Hormone-naïve Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur Urol 2018;73:834-44. [Crossref] [PubMed]
- Kassem L, Shohdy KS, Abdel-Rahman O. Abiraterone acetate/androgen deprivation therapy combination versus docetaxel/androgen deprivation therapy combination in advanced hormone sensitive prostate cancer: a network meta-analysis on safety and efficacy. Curr Med Res Opin 2018;34:903-10. [Crossref] [PubMed]
- Sydes MR, Spears MR, Mason MD, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol 2018;29:1235-48. [Crossref] [PubMed]
- Di Maio M, Bruzzi P, Perrone F, et al. Methodological issues in the choice among different drugs approved for the same therapeutic indication: a position paper by the Italian Association of Medical Oncology (AIOM). ESMO Open 2016;1:e000109 [Crossref] [PubMed]
- Tucci M, Leone G, Buttigliero C, et al. Hormonal treatment and quality of life of prostate cancer patients: new evidence. Minerva Urol Nefrol 2018;70:144-51. [PubMed]


