
Diagnostic value of glypican-3, arginase-1 and hepatocyte paraffin antigen -1 in differentiating hepatocellular carcinoma from intrahepatic cholangiocarcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, ranking fifth in frequency and the third leading cause of cancer-related mortality (1-3). The incidence of HCC has significantly increased in China; it accounts for 50% of the annual incidence of tumors and is the second cause of cancer-related deaths (4-6). The distinction of HCC from intrahepatic cholangiocarcinoma (ICC) and other types of metastatic adenocarcinoma to the liver is a challenge for its diagnosis and treatment (7). Some traditionally recognized hepatocyte-specific markers, including hepatocyte paraffin antigen 1 (HepPar-1), Golgi protein 73 (GP73), CD34, CD31, CD10 and α-fetoprotein, are considered to be insufficient for the diagnosis of HCC, particularly in cases of poorly differentiated HCC and metastatic tumors (8-11). Over the last two decades, it has become urgent to discover and evaluate new biomarkers for better initial and differential diagnosis of HCC. Several new biomarkers which highly expressed in HCC, including glypican-3 (GPC3) (12-16) and arginase-1 (Arg-1) (17-19), have been recognized as useful diagnostic biomarkers in differentiating HCC from metastatic tumors or ICC. However, the reported results were somehow inconsistent, and their value has not yet been confirmed.
In the present study, the diagnostic value of GPC3, Arg-1 and HepPar-1 in differentiating HCC from ICC was investigated by immunohistochemical staining, with a self-produced monoclonal antibody against GPC3 (20). Different combination models for these three biomarkers were also evaluated in terms of their ability to diagnose HCC and differentiated HCC from ICC.
Methods
Tissue specimens
This retrospective study included 47 cases of HCC (37 men and 10 women aged 28–82 years) and 29 cases of ICC (22 men and 7 women aged 40–78 years). The differentiation stages of the specimens are shown in Table 1. All cases were retrieved from the archives of the First Affiliated Hospital of Zhejiang University between 2012 and 2014. The clinical history, pathology reports and hematoxylin and eosin-stained slides for all cases were reviewed to confirm the diagnosis, according to the World Health Organization criteria (2008 edition) and the International Consensus Group for Hepatocellular Neoplasia in 2009 (21). Follow-up information of both recurrence and survival in HCC patients was collected as much as possible for nearly three-years. The study was approved by the Ethical Committee of the First Affiliated Hospital of Zhejiang University (REC number: EC-2015-82). The specimens were obtained with the consent of the patients and signed consent forms are kept in the medical Records Library.
Table 1
| Cancer | Well-differentiated | Moderately differentiated | Poorly differentiated | Total |
|---|---|---|---|---|
| HCC | 19 | 20 | 8 | 47 |
| ICC | 4 | 17 | 8 | 29 |
HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
Reagents and immunohistochemistry
Four-micron-thick sections of the formalin-fixed, paraffin-embedded tissue blocks of all the studied cases (including their paired paracancerous tissues) were prepared for immunohistochemistry targeted to GPC3, Arg-1 and HepPar-1. Immunohistochemistry was performed using the immunoperoxidase method. Positive control and negative control were set. In brief, sections were deparaffinized with xylene and rehydrated through a series of ethanol solutions. Heat-induced antigen retrieval was conducted in 0.1 mol/L citrate buffer (pH 6.0) in a microwave for 20 min. Endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 15 min. Pretreated sections were incubated with primary mouse monoclonal antibody against GPC3 (clone 7D11; 1:100 dilution; Darui Biotechnology, Guangzhou, China), HepPar-1 (clone OCH1E5; 1:200 dilution; DakoCytomation, Carpinteria, CA, USA) and Arg-1 (HPA003595; 1:200 dilution, Merck KGaA, Darmstadt, Germany) overnight at 4 °C. The reaction was detected with EnVision™ + Dual Link System-HRP (DAB) kit (Agilent Technologies, Inc., Santa Clara, CA, USA). Sections were counter stained with hematoxylin for 15 sec before being checked under a microscope. For the negative control, phosphate-buffered saline was substituted for the primary antibody.
Scoring of immunostaining
Semi-quantitative analysis was used to assess staining intensity and percentages of the cells. A four-tiered scale was introduced based on the intensity and the total percentage of positive cells as follows: Negative (no staining/weak staining and ≤10% stained), 1+ (weak staining but >10% stained, or dark staining with 5–10% stained), 2+ (moderate staining, and 10–50% stained), and 3+ (dark staining, and >50% stained). The Scoring of immunostaining work was done by two researchers independently. The inconsistent results were re-scored again.
Statistical analysis
The positive rate, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of Arg-1, HepPar-1 and GPC3 were analyzed for significance. Statistical analysis was performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA). Paired crosstabs were sorted out and McNemar’s test was done for the group comparison of Positive rate, sensitivity, or specificity. P<0.05 was set for statistically significant for each crosstab. When the compared groups (k) are three or more than three, the formula was used for the check level cutting. As for the comparison of the expression rate for each biomarker in different sample groups, Pearson Chi-square test (or Fisher’s Exact Test) was chosen. P<0.05 was set for statistically significant.
Results
GPC3, Arg-1 and HepPar-1 expression rate in different tissues
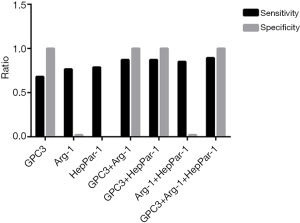
The immunostaining results (see Table 2) showed that GPC3, Arg-1 and HepPar-1 all had a higher expression rate in HCC tissues (68.09%, 76.60% and 78.72%, respectively) and lower in ICC tissues (6.90%, 6.90% and 13.79%, respectively) (P<0.05). There were no obvious difference of the expression rates between these three biomarkers both in HCC and in ICC (P>0.0125). No obvious difference in the expression distribution was observed between these three biomarkers in the different differentiated HCC. Therefore, the positive rate in poorly differentiated HCC for either GPC3 (62.50% vs. 73.68% and 65.00%), Arg-1 (62.50% vs. 73.68% and 85.00%) or HepPar-1 (62.50% vs. 78.95% and 85.00%), appeared to be slightly lower than that in well- and moderately differentiated HCC. With regard to specificity, GPC3 performed better than Arg-1 and HepPar-1 (97.37% vs. 1.32% and 2.63%), while there was no difference in specificity between Arg-1 and HepPar-1 (see Table 3 and Figure 1). Difference was observed among the expression rate of GPC3, Arg-1 and HepPar-1 in HCC, ICC and paracancerous tissues, respectively (P<0.05). Furthermore, sample BC99, which was originally classified as ICC, showed positive results for GPC3, Arg-1 and HepPar-1 and was re-evaluated as HCC.
Table 2
| Cancer kind | Total | GPC3 | Arg-1 | HepPar-1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| (−) | (+) | (−) | (+) | (−) | (+) | ||||
| HCC | 47 | 15 (31.91%) | 32 (68.09%) | 11 (23.40%) | 36 (76.60%) | 10 (21.28%) | 37 (78.72%) | ||
| Well-differentiated | 19 | 5 (26.32%) | 14 (73.68%) | 5 (26.32%) | 14 (73.68%) | 4 (21.05%) | 15 (78.95%) | ||
| Moderately differentiated | 20 | 7 (35.00%) | 13 (65.00%) | 3 (15.00%) | 17 (85.00%) | 3 (15.00%) | 17 (85.00%) | ||
| Poorly differentiated | 8 | 3 (37.50%) | 5 (62.50%) | 3 (37.50%) | 5 (62.50%) | 3 (37.50%) | 5 (62.50%) | ||
| ICC | 29 | 27 (93.10%) | 2 (6.90%) | 27 (93.10%) | 2 (6.90%) | 25 (86.21%) | 4 (13.79%) | ||
| Well-differentiated | 4 | 4 (100.00%) | 0 (0.00%) | 4 (100.00%) | 0 (0.00%) | 3 (75.00%) | 1 (25.00%) | ||
| Moderately differentiated | 17 | 16 (94.12%) | 1† (5.88%) | 15 (88.24%) | 2† (11.76%) | 15 (88.24%) | 2† (11.76%) | ||
| Poorly differentiated | 8 | 7 (87.50%) | 1 (12.50%) | 8 (100.00%) | 0 (0.00%) | 7 (87.50%) | 1 (12.50%) | ||
| Paracancerous tissue | 76 | 74 (97.37%) | 2 (2.63%) | 1 (1.32%) | 75 (98.68%) | 2 (2.63%) | 74 (97.37%) | ||
†, represented the sample BC99. GPC3, glypican-3; Arg-1, arginase-1; HepPar-1, hepatocyte paraffin antigen 1; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
Table 3
| Biomarker (s) | HCC (n=47), paracancerous tissue (n=47) | ICC (n=29) | |||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | (+) | (−) | ||
| GPC3 | 68.09% (32/47) | 100.00% (47/47) | 100.00% (32/32) | 75.81% (47/62) | 2 (6.90%) | 27 (93.10%) | |
| Arg-1 | 76.60% (36/47) | 2.13% (1/47) | 43.90% (36/82) | 8.33% (1/12) | 2 (6.90%) | 27 (93.10%) | |
| HepPar-1 | 78.72% (37/47) | 0.00% (0/47) | 44.05% (37/84) | 0.00% (0/10) | 4 (13.79%) | 25 (86.21%) | |
| GPC3 + Arg-1 | 87.23% (41/47) | 100.00% (47/47) | 100.00% (41/41) | 88.68% (47/53) | 1§ (3.45%) | 28 (96.55%) | |
| GPC3 + HepPar-1 | 87.23% (41/47) | 100.00% (47/47) | 100.00% (41/41) | 88.68% (47/53) | 1§ (3.45%) | 28 (96.55%) | |
| Arg-1 + HepPar-1 | 85.11% (40/47) | 2.13% (1/47) | 46.51% (40/86) | 12.50% (1/8) | 1§ (3.45%) | 28 (96.55%) | |
| GPC3 + Arg-1 + HepPar-1 | 89.36% (42/47) | 100.00% (47/47) | 100.00% (42/42) | 90.38% (47/52) | 1§ (3.45%) | 28 (96.55%) | |
§, represented the sample BC99. GPC3, glypican-3; Arg-1, arginase-1; HepPar-1, hepatocyte paraffin antigen 1; PPV, positive predictive value; NPV, negative predictive value; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.
GPC3, Arg-1 and HepPar-1 expression levels in different tissues
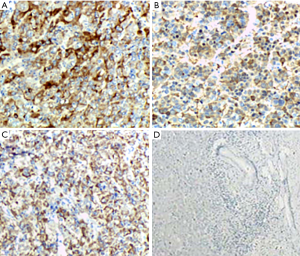
According to the scoring system, the expression level results of GPC3, Arg-1 and HepPar-1 are shown in Table 4. With regard to GPC3 and Arg-1, the majority of positive samples were grade 2+ in well- (52.63, 42.11%), moderately- (50.00, 60.00%) or poorly differentiated HCC (37.50, 62.50%), while the distribution of HepPar-1 was found slightly different (see Figure 2A,B,C). On the other hand, the expression intensity of HepPar-1 in poorly differentiated HCC seemed much higher than that of GPC3 and Arg-1 (40.00% vs. 0.00% and 0.00% for grade 3+).
Table 4
| Cancer kind | Total | GPC3 | Arg-1 | HepPar-1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (−) | 1+ | 2+ | 3+ | 0 (−) | 1+ | 2+ | 3+ | 0 (−) | 1+ | 2+ | 3+ | ||||
| HCC | 47 | 15 | 6 | 23 | 3 | 11 | 5 | 25 | 6 | 10 | 8 | 14 | 15 | ||
| Well-differentiated | 19 | 5 | 2 | 10 | 2 | 5 | 3 | 8 | 3 | 4 | 1 | 7 | 7 | ||
| Moderately differentiated | 20 | 7 | 2 | 10 | 1 | 3 | 2 | 12 | 3 | 3 | 5 | 6 | 6 | ||
| Poorly differentiated | 8 | 3 | 2 | 3 | 0 | 3 | 0 | 5 | 0 | 3 | 2 | 1 | 2 | ||
| ICC | 29 | 27 | 1 | 1 | 0 | 27 | 1 | 1 | 0 | 25 | 1 | 3 | 0 | ||
| Well-differentiated | 4 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | ||
| Moderately differentiated | 17 | 16 | 1‡ | 0 | 0 | 15 | 1 | 1‡ | 0 | 15 | 1 | 1‡ | 0 | ||
| Poorly differentiated | 8 | 7 | 0 | 1 | 0 | 8 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | ||
| Paracancerous tissue | 76 | 74 | 2 | 0 | 0 | 1 | 3 | 38 | 34 | 2 | 7 | 39 | 28 | ||
‡, represented the sample BC99. GPC3, glypican-3; Arg-1, arginase-1; HepPar-1, hepatocyte paraffin antigen 1; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma.

Diagnostic value of GPC3, Arg-1 and HepPar-1 in differentiating HCC from ICC
As shown in Table 4, since Arg-1 and HepPar-1 were recognized as hepatocyte-specific markers and not only specific for HCC, their PPVs lower than that of GPC3 in diagnosing HCC (43.90% and 44.05% vs. 100.00%, respectively; P<0.05). GPC3 had the best specificity for HCC (100%), along with a reasonable sensitivity (68.09%). GPC3 combined with Arg-1 or HepPar-1, or Arg-1 and HepPar-1, had an improved sensitivity (P=0.04, 0.04, 0.02; <0.05, respectively) and kept the well specificity in diagnosing HCC (see Figure 1). Meanwhile, in poorly differentiated HCC the positive number was increased only from 5 to 6 when GPC3 was combined with Arg-1 or HepPar-1, or Arg-1 and HepPar-1, and no difference was observed when Arg-1 was combined with HepPar-1. The interesting thing was that sample BC99, which positively expressing GPC3, Arg-1 and HepPar-1, was originally classified as ICC and later re-evaluated as HCC. Furthermore, 2 samples that tested negative for Arg-1 and HepPar-1, which were originally classified as HCC, were later re-evaluated as non-HCC.
The prognosis value of GPC3 for HCC patients
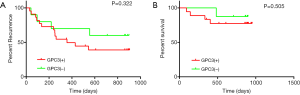
Follow-up information of both recurrence and survival in HCC patients was collected for nearly three-years, and 39 of 47 have been obtained. Among them, 7 were loss to follow-up for recurrence and 13 for survival. The recurrence rate in GPC3 positive group seemed little higher than that in GPC3 negative group (13/22 vs. 4/10), but no statistical significance was found (P=0.3158, >0.05). The analysis of DFS and OS also showed the same results (see Figure 3). There are 4 patients (4/18) reached the point of death in GPC3 positive group, while there is only one (1/8) in GPC3 negative group.
Discussion
In recent years, preliminary investigations of aberrant expression of GPC3, Arg-1 and HepPar-1 in HCC have been reported. Evidences prove that GPC3, Arg-1 and HepPar-1 plays important roles in progression and metastasis of HCC. Such as, GPC3 promotes proliferation and invasion of HCC (22,23). ARG1 might play a key role in progression of HCC via promoting the epithelial-to-mesenchymal transition (EMT) process (24). Suppression of the expression of Hep Par1 in liver cancer cells (SMMC-7721) inhibited cell proliferation (25). Collectively, these reports provide us a glimpse of dynamic involvements of GPC3, Arg-1 and HepPar-1 in HCC and may as potential biomarkers and therapeutic targets. Our follow-up results showed that the recurrence rate in GPC3 positive group seemed little higher than that in GPC3 negative group. Though no statistical significance was found, it may due to the small sample size and higher rate of loss to follow-up.
Distinction of HCC from non-hepatocellular-associated carcinoma is challenging, particularly in cases of poorly differentiated HCC. Various biomarkers, such as Arg-1, HepPar-1, CD34, GP73 and HSP70, have been proposed for distinguishing HCC from other types of liver cancer. However, none of them have been reported to be sufficient (26-28). Arg-1 and HepPar-1, the two most sensitive biomarkers for HCC (with a positive rate of 60–100%), have been turned up with a very poor specificity (29-31). GPC3, a member of the glypican family of heparin sulfate proteoglycans, has emerged as a new promising HCC diagnostic biomarker with positive expression rate of 70–90% and specificity of <90% (32-34). HepPar-1 is recognized as a traditional hepatocyte-specific marker. GPC3 and Arg-1 are relatively new diagnostic biomarkers that are highly expressed in HCC and considered useful in differentiating HCC from metastatic tumors or ICC. An increasing number of studies have reported GPC3 as a new HCC diagnostic biomarker both for early diagnosis or companion diagnosis, due to its advanced specificity and sensitivity. Immunostaining for GPC3, HSP70 and glutamine synthetase and/or gene expression (GPC3, LYVE1 and survivin) is recommended by the EASL-EORTC Clinical Practice Guidelines on the management of HCC for the differentiation between high grade dysplastic nodules and early HCC (35). In our study, the sensitivity and specificity of GPC3 was both superior to those of Arg-1 and HepPar-1. Furthermore, the combination of GPC3, Arg-1 and Par-1 had the best specificity and an improved sensitivity in diagnosing HCC and differentiating HCC from ICC. The aim of this study was to evaluate the diagnostic value of GPC3, Arg-1 and HepPar-1 in HCC, as well as in differentiating HCC from ICC. The results showed that both Arg-1 and HepPar-1 had a high positive expression rate (76.60% and 78.72%, respectively) but poor specificity (2.13% and 0.00%, respectively) for HCC. Even, the positive expression rate of Arg-1 and HepPar-1 in precancerous tissues (98.68% and 97.37%, respectively) was higher than that in HCC. It was consistent with previous reports (36,37). The high specificity of GPC3 and high sensitivity of Arg-1 and HepPar-1 led to the improvement of both sensitivity and specificity in the diagnosis of HCC when GPC3 was combined with Arg-1 or HepPar-1, or Arg-1 and HepPar-1. However, only a small increase of sensitivity was observed in the poorly differentiated HCC subgroup. The small sample size may be the reason for this limitation. It has been previously reported that the positive rate of GPC3 increased along with the decreased differential level in HCC (38,39). In the present study, the positive rate of GPC3 in poorly differentiated HCC was a slightly lower than that in moderate- or well-differentiated HCC. The same distribution trends were observed in Arg-1 and HepPar-1. It appears that all three biomarkers are insufficient for poorly differentiated HCC.
As for distinguishing HCC from ICC, all three biomarkers performed well. The combined panel of markers seems to have little improved effect in distinguishing HCC from ICC compared with single marker. Of note, sample BC99, which positively expressed GPC3, Arg-1 and HepPar-1, was originally classified as ICC and later re-evaluated as HCC. Furthermore, 2 samples that reported negative for Arg-1 and HepPar-1, which originally classified as HCC, were later re-evaluated as non-HCC. This may, or may not be a coincidence. The complete positive results were shown for all the three biomarkers (GPC3, Arg-1 and HepPar-1), and the PPV may indicate for HCC more accurate (PPV, 100%; Table 4). The diagnosis of ICC should be made carefully, and re-evaluation may be required. Conversely, negative results for both Arg-1 and HepPar-1 might suggest the possibility of non-HCCs. More consideration should therefore be taken in clinical practice. A limitation of this study was the insufficient statistical power derived from the small sample size. So here we presented the results descriptively without statistically significant. And we would prefer to present these results as phenomena or clues other than as conclusions. Studies with a larger sample size and more types of cancer may provide more information in searching for new biomarkers and new diagnostic methods (40,41).
The highlights of the present study were as follows: (I) the biomarkers examined in this study, particularly GPC3 and Arg-1in, are recently reported biomarkers in the pathological diagnosis of early HCC and differential diagnosis of HCC, cholangiocarcinoma and metastatic carcinoma of the liver (42,43). (II) The combination of GPC3, Arg-1 and HepPar-1 in differentiating HCC from ICC is a relatively new method that has not been sufficiently studied (12,15,19). Herein, the diagnostic and differential diagnostic values of these three markers in single or different combined styles were validated in different classified tissues, according to pathological results, respectively. The expression rate and level of GPC3, Arg-1 and HepPar-1 in different origin tissues (HCC, ICC, and their adjacent tissues) and different differentiated HCC tissues were all examined. It was found that the expression intensity of HepPar-1 in poorly differentiated HCC was much higher than that of GPC3 and Arg-1. (III) Since GPC3 is a relatively new biomarker, there is no standard monoclonal antibody for it. Even though the commercial monoclonal antibody 1G12 can be purchased and has been included in several studies, it does not mean that there are no better options. Yasuda et al. used a commercial ELISA kit with a GPC3 antibody 1G12 and reported that it did not perform well in diagnosing HCC (44). In the present study, the 7D11 monoclonal antibody 7D11 was used (our patent product, ZL201210086009.X, http://cpquery.sipo.gov.cn/) (20); it has been confirmed as an useful regent for GPC3 detecting by IHC (45), chemiluminescent immunoassay (41,46) and time-resolved fluorescence immunoassay (40). Our previous study showed that the 7D11 antibody was equivalent to 1G12 as a regent for GPC3 detection by IHC (45). The patent for the monoclonal antibody 7D11 was transferred to Darui Biotechnology, Guangzhou on Aug 23, 2017, and it is now available for commercialized use by Darui Biotechnology.
In conclusion, the comparison between GPC3 and Arg-1 or HepPar-1 suggested that the combination of GPC3, Arg-1 and Par-1 showed the best specificity and reasonable sensitivity for HCC. GPC3, Arg-1 and HepPar-1 were all useful biomarkers in differentiating HCC from ICC. The combination models can improve the values of any of these markers individually, both in diagnosing HCC and in differentiating HCC from ICC.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the First Affiliated Hospital of Zhejiang University (REC number: EC-2015-82). The specimens were obtained with the consent of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [Crossref] [PubMed]
- Islami F, Miller KD, Siegel RL, et al. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin 2017;67:273-89. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol 2015;13:594-601.e1. [Crossref] [PubMed]
- Hsiao JH, Tsai CC, Liang TJ, et al. Adjuvant hepatic arterial infusion chemotherapy is beneficial for selective patients with Hepatocellular carcinoma undergoing surgical treatment. Int J Surg 2017;45:35-41. [Crossref] [PubMed]
- Shen J, He L, Li C, et al. Nomograms to Predict the Individual Survival of Patients with Solitary Hepatocellular Carcinoma after Hepatectomy. Gut Liver 2017;11:684-92. [Crossref] [PubMed]
- Song P, Gao J, Inagaki Y, et al. Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and china. Liver Cancer 2013;2:31-9. [Crossref] [PubMed]
- Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 2016;22:9279-87. [Crossref] [PubMed]
- Lin D-C, Mayakonda A, Dinh HQ, et al. Genomic and Epigenomic Heterogeneity of Hepatocellular Carcinoma. Cancer research 2017;77:2255-65. [Crossref] [PubMed]
- Yao S, Zhang J, Chen H, et al. Diagnostic value of immunohistochemical staining of GP73, GPC3, DCP, CD34, CD31, and reticulin staining in hepatocellular carcinoma. J Histochem Cytochem 2013;61:639-48. [Crossref] [PubMed]
- Abdel-Hamid NM, Abouzied MM, Nazmy MH, et al. A suggested guiding panel of seromarkers for efficient discrimination between primary and secondary human hepatocarcinoma. Tumour Biol 2016;37:2539-46. [Crossref] [PubMed]
- Cao W, Sharma M, Imam R, et al. Study on Diagnostic Values of Astrocyte Elevated Gene 1 (AEG-1) and Glypican 3 (GPC-3) in Hepatocellular Carcinoma. Am J Clin Pathol 2019;152:647-655. [Crossref] [PubMed]
- El-Saadany S, El-Demerdash T, Helmy A, et al. Diagnostic Value of Glypican-3 for Hepatocellular Carcinomas. Asian Pac J Cancer Prev 2018;19:811-7. [PubMed]
- Montalbano M, Georgiadis J, Masterson AL, et al. Biology and function of glypican-3 as a candidate for early cancerous transformation of hepatocytes in hepatocellular carcinoma Oncol Rep 2017;37:1291-300. (Review). [Crossref] [PubMed]
- Pour AM, Masir N, Rose IM. Glypican-3 is useful but not superior to Hep Par 1 in differentiating hepatocellular carcinoma from other liver tumours. Malays J Pathol 2016;38:229-33. [PubMed]
- Han HH, Qiu YJ, Shi YY, et al. Glypican-3-targeted precision diagnosis of hepatocellular carcinoma on clinical sections with a supramolecular 2D imaging probe. Theranostics 2018;8:3268-74. [Crossref] [PubMed]
- Sin YY, Ballantyne LL, Richmond CR, et al. Transplantation of Gene-Edited Hepatocyte-like Cells Modestly Improves Survival of Arginase-1-Deficient Mice. Mol Ther Nucleic Acids 2018;10:122-30.
- Clark I, Shah SS, Moreira R, et al. A subset of well-differentiated hepatocellular carcinomas are Arginase-1 negative. Hum Pathol 2017;69:90-5. [Crossref] [PubMed]
- Geramizadeh B, Seirfar N. Diagnostic Value of Arginase-1 and Glypican-3 in Differential Diagnosis of Hepatocellular Carcinoma, Cholangiocarcinoma and Metastatic Carcinoma of Liver. Hepat Mon 2015;15:e30336. [Crossref] [PubMed]
- Ma RJ, Wang SH, Qin SN, et al. Preparation and characterization of monoclonal antibody against glypican-3. Hybridoma (Larchmt) 2012;31:455-61. [Crossref] [PubMed]
- Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 2009;49:658-64. [Crossref] [PubMed]
- Wang B, Xian J, Zang J, et al. Long non-coding RNA FENDRR inhibits proliferation and invasion of hepatocellular carcinoma by down-regulating glypican-3 expression. Biochemical and biophysical research communications. 2019;509:143-7. [Crossref] [PubMed]
- Capurro MI, Xiang YY, Lobe C, et al. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245-54. [Crossref] [PubMed]
- You J, Chen W, Chen J, et al. The Oncogenic Role of ARG1 in Progression and Metastasis of Hepatocellular Carcinoma. BioMed research international. vol. 2018, Article ID 2109865, 10 pages, 2018
- Yin YC, Li MJ, Sun XM, et al. Expression and role of Hep Par1 in hepatoma cells. Sciencepaper Online. [2010-02-09]
- Jing JS, Ye W, Jiang YK, et al. The Value of GPC3 and GP73 in Clinical Diagnosis of Hepatocellular Carcinoma. Clin Lab 2017;63:1903-9. [Crossref] [PubMed]
- Fujisaki S, Takashina M, Tomita R, et al. Treatments of Other Cancers and Liver Metastases after Hepatectomy for Liver Cancers. Gan To Kagaku Ryoho 2015;42:1674-6. [PubMed]
- Cui DJ, Wu Y, Wen DH. CD34, PCNA and CK19 expressions in AFP- hepatocellular carcinoma. Eur Rev Med Pharmacol Sci 2018;22:5200-5. [PubMed]
- Chen D, Li Z, Song Q, et al. Clinicopathological features and differential diagnosis of hepatocellular carcinoma in extrahepatic metastases. Medicine (Baltimore) 2018;97:e13356. [Crossref] [PubMed]
- Weiskirchen R. Intratumor heterogeneity, variability and plasticity: questioning the current concepts in classification and treatment of hepatocellular carcinoma. Hepatobiliary Surg Nutr 2016;5:183-7. [PubMed]
- Kim JU, Shariff MI, Crossey MM, et al. Hepatocellular carcinoma: Review of disease and tumor biomarkers. World J Hepatol 2016;8:471-84. [Crossref] [PubMed]
- Nishida T, Kataoka H. Glypican 3-Targeted Therapy in Hepatocellular Carcinoma. Cancers (Basel) 2019;11: [Crossref] [PubMed]
- Sai W, Wang L, Zheng W, et al. Abnormal Expression of Golgi Protein 73 in Clinical Values and Their Role in HBV-Related Hepatocellular Carcinoma Diagnosis and Prognosis. Hepat Mon 2015;15:e32918. [Crossref] [PubMed]
- Chen L, Chu F, Cao Y, et al. Serum miR-182 and miR-331-3p as diagnostic and prognostic markers in patients with hepatocellular carcinoma. Tumour Biol 2015;36:7439-47. [Crossref] [PubMed]
- EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Fujikura K, Yamasaki T, Otani K, et al. BSEP and MDR3: Useful Immunohistochemical Markers to Discriminate Hepatocellular Carcinomas From Intrahepatic Cholangiocarcinomas and Hepatoid Carcinomas. Am J Surg Pathol 2016;40:689-96. [Crossref] [PubMed]
- Fu LY, Mitchell KA, Cai G. Clear cell hepatocellular carcinoma diagnosed by bile duct brushing cytology. Diagn Cytopathol 2016;44:147-51. [Crossref] [PubMed]
- Wang P, Zhou X, Teng X, et al. Expressions and significance of Arginase-1,Glypican-3 and Hep Par-1 in hepatocellular carcinoma. Chin J Gastroenterol Hepatol 2017;26:390-394.
- Cheng L, Huang WB, Zhao YC, et al. Diagnostic value of the detection of Arg-1,HepPar-1,GPC3 and AFP in hepatocellular carcinoma. Chin J Diag Pathol 2015;22:598-605.
- Chen JJ, Xie CM, Wang CR, et al. Development of a Time-Resolved Fluorescence Immunoassay for the Diagnosis of Hepatocellular Carcinoma Based on the Detection of Glypican-3. J Fluoresc 2017;27:1479-85. [Crossref] [PubMed]
- Yu JP, Xu XG, Ma RJ, et al. Development of a clinical chemiluminescent immunoassay for serum GPC3 and simultaneous measurements alone with AFP and CK19 in diagnosis of hepatocellular carcinoma. J Clin Lab Anal 2015;29:85-93. [Crossref] [PubMed]
- McKnight R, Nassar A, Cohen C, et al. Arginase-1: a novel immunohistochemical marker of hepatocellular differentiation in fine needle aspiration cytology. Cancer Cytopathol 2012;120:223-9. [Crossref] [PubMed]
- Nassar A, Cohen C, Siddiqui MT. Utility of glypican-3 and survivin in differentiating hepatocellular carcinoma from benign and preneoplastic hepatic lesions and metastatic carcinomas in liver fine-needle aspiration biopsies. Diagn Cytopathol 2009;37:629-35. [Crossref] [PubMed]
- Yasuda E, Kumada T, Toyoda H, et al. Evaluation for clinical utility of GPC3, measured by a commercially available ELISA kit with Glypican-3 (GPC3) antibody, as a serological and histological marker for hepatocellular carcinoma. Hepatol Res 2010;40:477-85. [Crossref] [PubMed]
- Yu J, Ma Q, Zhang B, et al. Clinical application of specific antibody against glypican-3 for hepatocellular carcinoma diagnosis. Sci China Life Sci 2013;56:234-9. [Crossref] [PubMed]
- Xie C, Tiede C, Zhang X, et al. Development of an Affimer-antibody combined immunological diagnosis kit for glypican-3. Sci Rep 2017;7:9608. [Crossref] [PubMed]


