
Postoperative carcinoembryonic antigen (CEA) levels predict outcomes after resection of colorectal cancer in patients with normal preoperative CEA levels
Introduction
Colorectal cancer (CRC) is currently the third most common cancer and one of the major causes of cancer-related deaths worldwide (1). In recent years, the prevalence of CRC has increased rapidly in China (2). Despite extensive advances in adjuvant therapies and biomarker target drugs, the 5-year overall survival (OS) of CRC patients is still poor. Currently, resection of the primary tumors is the cornerstone of CRC management and is the only available treatment choice that offers the opportunity for a cure. Unfortunately, almost half of the CRC patients who underwent surgical resection died due to recurrences or metastatic diseases (3). Numerous studies have indicated that prognostic assessment mainly relies on clinical or pathological stages. Practically, however, the outcome of the same stage patients can be considerably different (4). Patients with other aggressive prognostic factors, e.g., elevated expression of CEA, vascular invasion, nerve infiltration, and positive surgical margin (5), would have a worse prognosis. Currently, postoperative monitoring is based on specific computed tomography (CT) or magnetic resonance imaging (MRI) exams. However, long-term survival was not always associated with radiologic data as this data lacked a predictive effect.
Carcinoembryonic antigen (CEA) is a sensitive cancer biomarker for gastrointestinal tumors and is widely expressed in CRC tissue and serum. Serum CEA has been commonly used in clinical practice since 1965 (6). As a classical marker for this disease, vast of studies have extensively assessed the prognostic role of serum CEA levels in CRC. In practice, serum CEA levels are widely used for posttreatment surveillance because it is easily detected without radiation injury. Vast reports have shown that the preoperative CEA level is an independent predictive factor of unfavorable prognosis for CRC (7,8). Some studies showed that CEA levels independently predict poor chemotherapy response. Most reports investigated the relation between serum CEA level and chemotherapy based on the preoperative level, and the clinical significance of the CEA value was barely studied among the patients whose CEA level was normal before surgery.
However, increasingly, investigators recognized that the CEA measurement lacked sensitivity and specificity in CRC surveillance (9). Therefore, some previous studies have redefined the CEA classification or combined CEA with other biomarkers for more accurate monitoring (10,11). Saito et al. found that the diagnostic accuracy of postoperative CEA levels for recurrence in patients with normal preoperative CEA levels was significantly higher than that among the population with positive preoperative serum CEA levels (12). Limited data assessed the diagnostic value and cutoff level of CEA among the population with normal preoperative CEA levels but elevated CEA levels after resection. Hence, in the present study, we sought to determine the clinical significance of the postoperative CEA levels in CRC patients who had normal CEA levels before surgery.
Methods
In this study, the clinical and pathological materials were based on the databases of Sir Run Run Shaw Hospital of Zhejiang University. A total of 1,492 patients diagnosed with CRC and who underwent R0 surgery between 2012 and 2016 were included in this search, among which 110 patients had a normal preoperative CEA level of 5 ng/mL or less and then subsequently developed an elevated CEA level at least one time. Among these patients, 16 patients were excluded because they had distant metastasis at the time of resection, histology that was not adenocarcinoma or unknown follow-up information. A total of 94 patients with p-stage I to p-stage III CRC were enrolled in the present study according to the American Joint Committee on Cancer (AJCC) eighth edition. All the therapies of these patients were based on the corresponding National Comprehensive Cancer Network (NCCN) guidelines. The clinicopathologic baseline features and follow-up data of each patient were collected. This study obtained approval from the Institutional Review Board, and the data were analyzed anonymously.
The serum levels of all 94 patients were measured every 3–6 months after surgery. All blood samples were sent to the Clinical Laboratory at Sir Run Run Shaw Hospital and analyzed using Unicel DxI800 (Beckman Coulter, Fullerton, CA, USA) for the detection of CEA. According to the postoperative serum CEA peak levels and variation trends, the patients were divided into different groups as follows: Group A1: the peak CEA level was no more than 10 ng/mL; Group A2: the peak CEA level was higher than 10 ng/mL; Group B1: the CEA level reached a normal level at least once during follow-up; and Group B2: the patients had persistently elevated CEA levels. This classification was defined as the CEA-stage. Additionally, we combined the TNM stage with the CEA-stage as follows: stage I-A1, stage I-A2, stage I-B1, stage I-B2 and so on. The primary end points of this study were disease-free survival (DFS) and OS. For each patient, recurrences and metastases were diagnosed by CT imaging examinations. DFS was measured from the surgical date to the time of recurrence (or death) or until the date of the last follow-up in patients who maintained a disease-free status. OS was calculated from the date of surgical resection to the time of death or deadline in survivors. The median follow-up time was 38 months.
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics 23 (IBM Corp., NY, USA). Differences in clinicopathological factors according to CEA levels were analyzed for all patients. Chi-squared or Fisher’s exact test was performed to compare the categorical characteristics of the groups. Student’s t-test was selected to examine the differences in continuous data. The DFS and OS of each group were assessed using the Kaplan-Meier curve. In addition, Cox’s proportional hazards regression model was used to identify the effect of the independent prognostic predictor on OS. Survival outcomes between groups were compared with the log-rank test. A P value of less than 0.05 derived from two-sided tests was considered statistically significant.
Results
Patient characteristics
In the present study, CRC patients with a normal preoperative CEA level that subsequently developed an elevated CEA level were observed in 6.30% colorectal patients. The majority of the included patients were stage II (30.9%) and stage III (58.5%) cases. The median age of these 94 patients was 63 years old (range, 31–94 years old). Most of the included patients were male (70.2%). Up to the date of the last follow-up, 48 (51.1%) patients had died, and 59 (62.8%) patients were diagnosed with CRC recurrence or distant metastasis.
Recurrence and metastasis according to clinicopathology
Patients in Group A2 and Group B2 had greater opportunities for recurrence and metastasis than Group A1 and Group B1, respectively (P<0.05). The baseline characteristics of all the 94 patients are summarized in Table 1. The data showed that the median peak level of CEA among all patients was 8.26 ng/mL (range, 5.03–2,879.00 ng/mL), and the average levels of peak CEA in Group A1 and Group A2 were 6.570 ng/mL and 141.543 ng/mL, respectively, but there were no statistically significant differences between these two groups (P>0.05). Interestingly, it was harder for rectal cancer patients to regain normal postoperative CEA levels than colon cancer patients (P<0.05). A Chi-squared analysis showed that peak CEA level higher than 10 ng/mL (P<0.05), trend of CEA level (P<0.05), invasion depth (P<0.05), and node metastasis (P<0.05) after resection were significant independent predictors of relapse (Table 2). No significant differences were found among age, sex, or tumor location after curative resection.
Table 1
| Variable | No. patients | Serum CEA level | Trend of CEA | |||||
|---|---|---|---|---|---|---|---|---|
| Group A1 | Group A2 | P value | Group B1 | Group B2 | P value | |||
| Sex | ||||||||
| Male | 66 | 39 | 27 | 0.883 | 24 | 42 | 0.121 | |
| Female | 28 | 17 | 11 | 15 | 13 | |||
| Age (years) | 61.989 | 61.357 | 62.921 | 0.529 | 61.641 | 62.236 | 0.3 | |
| Peak CEA level | 61.133 | 6.57 | 141.543 | 0.093 | 14.672 | 94.079 | 0.153 | |
| Tumor size (mm) | 38.0 | 35.929 | 41.053 | 0.176 | 36 | 39.418 | 0.60 | |
| T category | ||||||||
| T1, T2 | 11 | 9 | 2 | 0.113 | 6 | 5 | 0.403 | |
| T3, T4 | 80 | 44 | 36 | 33 | 47 | |||
| Lymphatic invasion | ||||||||
| Absence | 40 | 28 | 12 | 0.076 | 15 | 25 | 0.499 | |
| Presence | 54 | 28 | 26 | 24 | 30 | |||
| Location | ||||||||
| Colon | 41 | 22 | 19 | 0.304 | 22 | 19 | 0.035* | |
| Rectum | 53 | 17 | 36 | 17 | 36 | |||
| Recurrence and metastasis | ||||||||
| Absence | 35 | 31 | 4 | 0.000* | 23 | 12 | 0.000* | |
| Presence | 59 | 25 | 34 | 16 | 43 | |||
*, statistically significant. CRC, colorectal cancer; CEA, carcinoembryonic antigen.
Table 2
| Recurrence and metastasis | No. patients | Peak CEA | Trend of CEA | Deep of invasion | Node metastasis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A1 | Group A2 | P value | Group B1 | Group B2 | P value | T1,2 | T3,4 | P value | Positive | Negative | P value | |||||
| Yes | 35 | 31 | 4 | 0.000* | 23 | 12 | 0.000* | 11 | 24 | 0.000* | 20 | 15 | 0.028* | |||
| No | 59 | 25 | 34 | 16 | 43 | 2 | 57 | 20 | 39 | |||||||
*, statistically significant. CRC, colorectal cancer; CEA, carcinoembryonic antigen.
Evaluation of prognosis using the TNM-CEA-stage system
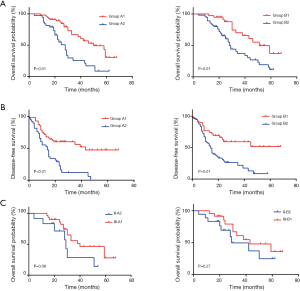
Using the Cox regression model, we found that high CEA levels (HR =2.993, 95% CI, 1.600–5.598) and consistently elevated CEA levels (HR =2.914, 95% CI, 1.475–5.756) were significantly associated with worse OS. The same results were seen in DFS (HR =1.949, 95% CI, 1.106–3.433 and HR =2.534, 95% CI, 1.322–4.859, respectively) (Table 3). As shown in Figure 1A, the Kaplan-Meier curve indicated that patients in Group B2 had a worse prognosis than patients in Group B1 (median survival 28 vs. 51 months, P<0.01). The same result was seen between Groups A2 and A1 (median survival 28 vs. 50 months, P<0.01) (Figure 1B). Poor DFS was also detected in Groups B2 and A2 compared to Groups B1 and A1, respectively (P<0.01). To further assess the prognostic roles of CEA levels, we established the prognostic values of the TNM-CEA-stage system. Though there were no significant differences, patients with stage II CRC in Group A2 demonstrated worse OS compared with the stage III-A1 patients (median survival 29 vs. 40 months, P=0.08). This result was consistent with the comparison between stages II-B2 and III-B1, which indicated that the prognosis of stage II patients with persistently elevated CEA levels was worse than that of stage III patients (median survival 29 vs. 43 months, P=0.27) (Figure 1C).
Table 3
| Variables | OS | DFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | |||||
| Female | 1.031 (0.521–2.039) | 0.931 | 1.171 (0.655–2.094) | 0.595 | |
| Male | Refa | ||||
| Peak CEA level | 0.001* | 1.949 (1.106–3.433) | 0.021* | ||
| Group A2 | 2.993 (1.600–5.598) | ||||
| Group A1 | Refa | ||||
| Trend of CEA | 0.002* | 2.534 (1.322–4.859) | 0.005* | ||
| Group B2 | 2.914 (1.475–5.756) | ||||
| Group B1 | Refa | ||||
| T stage | 0.766 | 4.338 (1.009–18.651) | 0.049* | ||
| T3,4 | 1.212 (0.342–4.293) | ||||
| T1,2 | Refa | ||||
| Lymph nodes | 0.433 | 1.487 (0.855–2.585) | 0.160 | ||
| Positive | 1.295 (0.679–2.473) | ||||
| Negative | Refa | ||||
| Location | 0.070 | 0.826 (0.473–1.443) | 0.502 | ||
| Rectum | 0.558 (0.297–1.048) | ||||
| Colon | Refa | ||||
a, reference; *, statistically significant. CEA, carcinoembryonic antigen; OS, overall survival; DFS, disease-free survival.
Discussion
After curative resection of primary CRC, a large number of patients’ CEA levels declined to normal values within 4–6 weeks. Residual disease should be suspected if the CEA level does not return to normal. Furthermore, the patients with persistent elevation of CEA levels following surgery could suggest worse survival (13), which might mean that an increased amount of CEA secreted was associated with greater tumor load. However, 49% of patients had a false increase of CEA at least once in follow-up, and more than 90% of these had values ranging from 5 to 10 ng/mL (14). In this retrospective study, we defined the cutoff as 10 ng/mL. One report by Moertel et al. (15) showed a false-positive rate of 4% when a cutoff of 10 ng/mL was used. Our results showed that only 4 patients remained disease-free until death once the CEA level was higher than 10 ng/mL.
Although other study (16) suggested that CRC patients with an increase in postoperative CEA levels were also associated with aggressive characteristics, including poor T stage and differentiation, there were no significant differences in basic clinicopathological features between CRC patients in Groups A1 and A2. When the comparison was made between the patients in Groups B1 and B2, the same conclusion was drawn. These results showed that the relationship between basic features and the preoperative CEA level was tighter than postoperative CEA levels, which was in accordance with previous studies.
In practice, CRC is mainly classified according to the TNM stage system, including the local invasion depth (T stage), the lymph node invasion (N stage), and the presence of distant metastases (M stage) (17). The TNM stage system has provided valuable prognostic messages and guided therapy. Regarding the pathological features at the time of diagnosis, the CRC was classified into early stage I disease (T1-T2, N0, and M0), stage II disease (T3-T4, N0, and M0), stage III disease (any T, N1-2, and M0), and advanced stage IV disease (any T, any N, and M1). The current recommended treatment for stages I, II, and III is surgical resection, and stage III patients are advised to receive adjuvant chemotherapy (18). For stage II patients, adjuvant chemotherapy is only recommended for patients with high-risk factors (19-21), but a definite consensus for this treatment has not yet been reached. In fact, the responses of systemic adjuvant chemotherapy for stage II and III patients were not predicted. Therefore, there is special attention and controversy regarding stages II and III disease.
Even with the advancement of chemotherapy regimens and targeted therapies, among patients with stages II and III disease, the survival rates were 62.1 and 36.5%, respectively, in the present study. The survival above was worse than the same stage CRC patients in a large population, which was consistent with epidemiological investigations that corroborated that high postoperative serum CEA levels were a significantly increased risk for CRC patients (22). Our study showed that both the patients in Groups A2 and B2 had a shorter OS than those in Groups A1 and B1, and the results were significantly different (P<0.05). Additionally, the prognosis of both Groups A2 and B2 was worse than that of Groups A1 and B1 with the same TNM stage. Thus, our data suggested that postoperative CEA level is an important prognostic marker in stage II/III CRC patients. The COX regression analysis showed that the CEA level or the trend was also a dependent risk factor for recurrence and metastasis. Either Group A2 or B2 accounted for a relatively higher proportion of relapse and metastasis in stage II/III CRC patients than Group A1 or B1. In our study, 89.5% of patients in Group A2 had developed relapse or metastasis. Therefore, irrespective of the stage of cancer, both the high level and the increasing trend of the postoperative CEA level determined poor OS in CRC.
Thus, for patients with elevated postoperative CEA levels, the current TNM staging system may not be enough to evaluate the prognosis and clinical outcomes. In this study, we examined the prognostic role of the level of the postoperative CEA serum biomarker combined with the TNM staging system in those patients. In addition, adding the CEA information to the TNM staging system indeed improved the survival predictive value compared with the TNM stage system alone. Interestingly, the OS of stage II-B2 patients was worse than that of stage III-B1 patients. In the future, the trend of postoperative CEA levels has the potential to serve as a marker in disease prognosis and may be a predictor of chemotherapy response. Similar results were drawn in the survival competition of stage II-A2 and stage III-A1 patients. As a result, among stage II patients with high CEA levels after surgery could be eligible patients for adjuvant chemotherapy and other therapies.
In clinical practice, patients came to hospital for a check-up every 3 months in the first 2 years and then every 6 months for a total of 5 years. Routine monitoring of the CEA level was suggested for all the patients with resected CRC. For this subgroup of CRC patients in our investigation, we recommended that the serum CEA level be surveilled more intensively once the level was greater than 10 ng/mL during follow-up. In particular, the use of serum protein biomarkers was noninvasive and inexpensive for the detection of CRC compared to a CT scan. Each follow-up, blood tumor markers and rectal examination should be conducted to assist oncologists in the early discovery of possible local recurrence and metastasis. Our study found that it was unlikely for the postoperative CEA levels for rectal cancer patients to be normal again. This information was potentially useful for doctors in terms of putting more comprehensive examination to detect disease progression.
A large number of reports demonstrated that the change in imaging tests was secondary to serum markers. The CEA level detected recurrent disease 2 to 5 months prior to discovery by any other means (23,24). Any rise in CEA level higher than 10 ng/mL should prompt thorough assessment for disease recurrence; however, false-positive elevated CEA levels have been reported to occur in patients who are smokers and patients with inflammatory states, including chronic obstructive lung diseases (25) and rheumatoid arthritis (26). In addition, the CEA level is not specific to CRC and could be elevated in many other cancers. Therefore, the multiphase influences resulting in CEA variation were one of the weaknesses of CEA to be used to guide the treatment and prognosis of CRC.
There were other limitations in the present study. This was a retrospective study with a relatively small sample size, which might be responsible for some negative results in terms of clinical characteristics or OS in different groups. The lack of pathology confirmed that recurrence or metastasis was also a potential limitation.
Conclusions
In conclusion, the present study revealed a new predictive method of serum CEA levels as a possible prognostic marker in colorectal tumors, especially for stage II patients during the following-up period. Among patients with an initially normal CEA level, CEA monitoring was found to be effective for assessing tumor progression. Hence, a personal posttreatment surveillance plan is needed for CRC patients to receive effective treatment.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study obtained ethics approval of Sir Run Run Shaw Hospital (No. 20190814-4). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Manfredi S, Bouvier AM, Lepage C, et al. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg 2006;93:1115-22. [Crossref] [PubMed]
- Jen J, Kim H, Piantadosi S, et al. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med 1994;331:213-21. [Crossref] [PubMed]
- Emre A, Akbulut S, Sertkaya M, et al. Assessment of risk factors affecting mortality in patients with colorectal cancer. Prz Gastroenterol 2018;13:109-17. [Crossref] [PubMed]
- Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med 1965;121:439-62. [Crossref] [PubMed]
- Cai Z, Xiao J, He X, et al. Accessing new prognostic significance of preoperative carcinoembryonic antigen in colorectal cancer receiving tumor resection: More than positive and negative. Cancer Biomark 2017;19:161-8. [Crossref] [PubMed]
- Kim IH, Lee JE, Yang JH, et al. Clinical significance of changes in systemic inflammatory markers and carcinoembryonic antigen levels in predicting metastatic colorectal cancer prognosis and chemotherapy response. Asia Pac J Clin Oncol 2018;14:239-46. [Crossref] [PubMed]
- Zhu J, Dong H, Zhang Q, et al. Combined assays for serum carcinoembryonic antigen and microRNA-17-3p offer improved diagnostic potential for stage I/II colon cancer. Mol Clin Oncol 2015;3:1315-8. [Crossref] [PubMed]
- Wu F, Chen L, Wu W, et al. Predictive value of combination detection of tissue Pgp1 expression and preoperative serum CEA level for colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2017;20:443-9. [PubMed]
- Gao Y, Wang J, Zhou Y, et al. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci Rep 2018;8:2732. [Crossref] [PubMed]
- Saito G, Sadahiro S, Kamata H, et al. Monitoring of Serum Carcinoembryonic Antigen Levels after Curative Resection of Colon Cancer: Cutoff Values Determined according to Preoperative Levels Enhance the Diagnostic Accuracy for Recurrence. Oncology 2017;92:276-82. [Crossref] [PubMed]
- Lin JK, Lin CC, Yang SH, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis 2011;26:1135-41. [Crossref] [PubMed]
- Litvak A, Cercek A, Segal N, et al. False-positive elevations of carcinoembryonic antigen in patients with a history of resected colorectal cancer. J Natl Compr Canc Netw 2014;12:907-13. [Crossref] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA 1993;270:943-7. [Crossref] [PubMed]
- Bhatti I, Patel M, Dennison AR, et al. Utility of postoperative CEA for surveillance of recurrence after resection of primary colorectal cancer. Int J Surg 2015;16:123-128. [Crossref] [PubMed]
- Doescher J, Veit JA, Hoffmann TK. The 8th edition of the AJCC Cancer Staging Manual: Updates in otorhinolaryngology, head and neck surgery. HNO 2017;65:956-61.
- Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005;23:8671-8. [Crossref] [PubMed]
- Benson AB 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408-19. [Crossref] [PubMed]
- Engstrom PF, Arnoletti JP, Benson AB 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw 2009;7:778-831. [Crossref] [PubMed]
- Labianca R, Nordlinger B, Beretta GD, et al. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2010;21:v70-7. [Crossref] [PubMed]
- Yang KM, Park IJ, Kim CW, et al. The prognostic significance and treatment modality for elevated pre- and postoperative serum CEA in colorectal cancer patients. Ann Surg Treat Res 2016;91:165-71. [Crossref] [PubMed]
- Hine KR, Dykes PW. Serum CEA testing in the post-operative surveillance of colorectal carcinoma. Br J Cancer 1984;49:689-93. [Crossref] [PubMed]
- McCall JL, Black RB, Rich CA, et al. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum 1994;37:875-81. [Crossref] [PubMed]
- Bulut I, Arbak P, Coskun A, et al. Comparison of serum CA 19.9, CA 125 and CEA levels with severity of chronic obstructive pulmonary disease. Med Princ Pract 2009;18:289-93. [Crossref] [PubMed]
- Koch MC, Pereira IA, Nobre LF, et al. Computed tomography of pulmonary changes in rheumatoid arthritis: carcinoembryonic antigen (CEA) as a marker of airway disease. Rheumatol Int 2016;36:531-9. [Crossref] [PubMed]


