
Genetic variations of rs6928 and rs5999521 of ERK2 were found to have correlation with the risk of brain metastasis in patients with lung adenocarcinoma
Introduction
The brain is one of the most common sites of distant metastasis of non-small cell lung cancer (NSCLC). Up to 40% of patients with NSCLC develop brain metastasis (BM) during disease progression (1-3). Notably, most drugs cannot penetrate the blood-brain barrier to eliminate possible residual diseases in brain, rendering the central nervous system a potential site of disease relapse, particularly in patients with a long life expectancy. BM has serious effects on patient quality of life (QOL) and can cause the mass effect and neurological dysfunction owing to intracranial lesions, resulting in reduced QOL. Most patients present with significant neurological signs and symptoms that are related to the location and extent of brain involvement, including both focal neurological changes and general symptoms secondary to increased intracranial pressure. Furthermore, patients with BM have a poor prognosis. Without treatment, the median overall survival (OS) is only 4–7 weeks (4,5). Even after whole-brain radiation therapy, the primary therapeutic approach for BM, the median OS was only extended to 3–5 months (6,7).
To date, no effective prophylactic measure has been established in patients with NSCLC. Prophylactic cranial irradiation (PCI) is the standard care for patients with small cell lung cancer (SCLC) who respond to initial treatments (8,9). In selected patients achieving either a complete or partial response, PCI has been shown to not only decrease the incidence of BM but also to improve long-term survival (10,11). Similar studies have been conducted in patients with NSCLC (1,12). However, only the incidence of BM was shown to be decreased by PCI, and no survival advantage was observed. One likely explanation for this difference is the heterogeneous risk of BM across all patients with NSCLC. Thus, the identification of patients at risk of BM could help inform the design of future studies to investigate preventative measures, such as PCI.
In various studies, clinical factors such as histology, lymph node metastasis (number or volume), age, the use of neoadjuvant chemotherapy, and prolonged survival were found to be associated with the increased incidence of brain metastases (3,13,14). However, some factors were subjective and/or difficult to quantify, which limited their use in clinical practice. SNPs have been widely used in the study of cancer susceptibility. Some researchers employed SNPs to explore the risk of metastasis (15,16). Thus, genetic markers are potentially promising factors for BM prediction.
Many studies have demonstrated that the incidence of BM in patients with adenocarcinoma is higher than that in other subtypes (3,14). Additionally, epidermal growth factor receptor (EGFR), which is frequently mutated in patients with adenocarcinoma, has been found to be associated with the risk of BM (17,18). Furthermore, as one of the most important downstream signalling pathways, mitogen-activated protein kinase (MAPK) was found to be an important mediator of EGFR/MET crosstalk, which was believed to be involved in the molecular mechanisms of BM (19).
Accordingly, in this study, we hypothesised that genetic variations in MAPK may be correlated with the risk of BM in patients with adenocarcinoma. To verify this hypothesis, we analysed single nucleotide polymorphisms (SNPs) in the MAPK gene in patients with adenocarcinoma.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-3069).
Methods
Patients
Patients with pathologically confirmed adenocarcinoma of the lung was eligible for the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review boards and ethics committees of Beijing Tiantan Hospital of Capital Medical University (KYSB2010-170-01) and The First Hospital of Jilin University (2011-031) and informed consent was taken from all the patients.
Treatments
At the time of diagnosis, peripheral blood samples were obtained from eligible patients for analysis. Before treatment, all patients underwent a baseline evaluation, including medical history, general physical examination, complete blood count, serum chemistry, and radiographic examinations. The treatment strategy was determined according to the stage of disease at diagnosis (American Joint Committee on Cancer, 6th ed.). Platinum-based chemotherapy was recommended postoperatively for patients with operable stage IB to IIIA disease. For patients with stage IIIB or IV disease, systemic therapy was considered. Definitive radiation therapy of 60 Gy/30 f, which was delivered using three-dimensional conformal radiation therapy and intensity modulated radiation therapy, was reserved for patients with stage I to III disease who were ineligible for surgical intervention (inadequate organ function or patient refusal). Concomitant systemic therapy was allowed if there were no contraindications. Patients receiving immune checkpoint inhibitors were excluded from the current study.
Follow-up
Baseline assessments were repeated at the completion of the planned therapy and were then repeated every 3–6 months for the first 2 years and every 6–12 months for the next 3 years. At the time of diagnosis, magnetic resonance imaging (MRI) was routinely performed for all patients. During the follow-up period, MRI was conducted every 3–6 months. If symptoms of BM were present during the follow-up interval, MRI was performed immediately.
SNP selection and genotyping
A haplotype tag SNP (htSNP) approach was utilised to analyse MAPK genetic polymorphisms globally. Genotyped HapMap SNPs among Han Chinese patients with a multiple allele frequency of greater than 5% were included in the selection (Table 1). MAPK SNPs were genotyped using the MassArray system (Sequenom). To assess reproducibility, 5% of the DNA samples were blindly and randomly analysed in duplicates, and the reproducibility was 99%. The sequences of primers and probes for each SNP are available upon request.
Table 1
| No. | MEK1 | Allelic change | MEK2 | Allelic change | ERK1 | Allelic change | ERK2 | Allelic change |
|---|---|---|---|---|---|---|---|---|
| 1 | rs11071888 | A>C | rs10250 | G>A | rs28529403 | T>C | rs9610505 | A>G |
| 2 | rs67930309 | A>C | rs6629 | G>A | rs61764202 | C>T | rs9607340 | T>G |
| 3 | rs1549854 | A>C | rs145934591 | TTT>TT | ||||
| 4 | rs12050732 | A>C | rs6928 | C>G | ||||
| 5 | rs9610417 | C>T | ||||||
| 6 | rs1063311 | C>T | ||||||
| 7 | rs8136867 | G>A | ||||||
| 8 | rs5999521 | A>G | ||||||
| 9 | rs2266966 | T>C |
EGFR mutation testing
Mutation analysis was conducted separately at the two hospitals and confirmed centrally at Beijing Tiantan Hospital. EGFR mutations were analysed in paraffin-embedded tissue sections. Tumour tissue was scraped from the glass slides under direct visualisation or under a dissecting microscope. DNA was then extracted using a QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA, USA). EGFR mutations were detected with an ADx EGFR Mutations Detection Kit (Amoy Diagnostics, Xiamen, China), which employs an amplification refractory mutation system. This assay was performed according to the manufacturer’s protocol using an ABI 7500 real-time polymerase chain reaction system (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
SPSS 22.0 software (SPSS Inc., Armonk, NY, USA) was used for statistical analysis. The chi-squared test was used to analyse differences between subgroups which were stratified by clinical factors. Logistic regression and Cox regression were conducted for multivariate analysis to evaluate the association of genetic variation with the risk of BM, adjusted for age, sex, staging, smoking status, surgery, and thoracic radiotherapy. Results with P values of less than 0.05 were used as the criterion of statistical significance, and all statistical tests were two sided.
Results
Patients’ characteristics
Between March 2010 and April 2015, 333 patients were eligible for this study; 120 were from Beijing Tiantan Hospital (discovery cohort), and 213 were from The First Hospital of Jilin University (validation cohort). The patients’ characteristics are summarised in Table 2. The median age was 61 years in the discovery cohort, which was significantly older than that (56 years) in the validation cohort. Additionally, in the discovery cohort, the proportion of patients with advanced disease was significantly lower than that in the validation cohort. As a result, thoracic surgery or thoracic radiotherapy, as prior options for early-stage disease, was applied more frequently in the discovery cohort than in the validation cohort. However, the differences were not significant.
Table 2
| Variables | Beijing cohort (n=120) | Changchun cohort (n=213) | P value | *Cox regression analysis | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | ||||
| Age (range) | 61 years (25–86 years) | 56 years (35–81 years) | <0.001 | 0.983 | 0.965–1.001 | 0.063 |
| Sex | 0.66 | 1.064 | 0.681–1.664 | 0.785 | ||
| Male | 69 (57.5) | 131 (61.5) | ||||
| Female | 51 (42.5) | 82 (38.5) | ||||
| Smoking status | 0.76 | 1.003 | 0.628–1.602 | 0.991 | ||
| Never smoked | 78 (65.0) | 132 (62.0) | ||||
| Current smokers | 42 (35.0) | 81 (38.0) | ||||
| Thoracic surgery | 0.19 | 1.062 | 0.628–1.796 | 0.822 | ||
| No | 69 (57.5) | 143 (67.1) | ||||
| Yes | 51 (42.5) | 70 (32.9) | ||||
| Thoracic radiation | 0.11 | 1.431 | 0.916–2.237 | 0.116 | ||
| No | 80 (66.7) | 167 (78.4) | ||||
| Yes | 40 (33.3) | 46 (21.6) | ||||
| Staging | 0.024 | 1.240 | 0.945–1.626 | 0.121 | ||
| I | 13 (10.8) | 17 (8.0) | ||||
| II | 20 (16.7) | 19 (8.9) | ||||
| III | 47 (39.2) | 62 (29.1) | ||||
| IV | 40 (33.3) | 115 (54.0) | ||||
| Brain metastasis | 55 (45.8) | 41 (19.2) | – | – | – | – |
*Regression analyses were conducted in all patients both from Beijing cohort and Changchun cohort. CI, confidence interval; HR, hazard ratio.
For all 333 patients, the median follow-up time was 24 months (range: 1–148 months). During that period, 96 patients had documented BM, with a median time to BM of 20 months (range: 1–145 months). In Cox regression analysis, only age was found to have marginal significance in terms of correlation with the risk of BM development. Those who were older at the time of diagnosis tended to have a lower risk of BM development [hazard ratio (HR): 0.983, P=0.063]. Otherwise, statistical analysis did not find other clinical factors to have associations with the risk of BM development.
Association between individual SNPs and risk of BM
In order to explore the potential associations of genetic variations with the risk of BM development, a multivariate Cox model was used. Initially, analyses were conducted in the discovery cohort. Of 17 selected individual SNPs, we found that the extracellular signal-regulated kinase 2 (ERK2) rs6928 and rs5999521 SNPs were associated with the risk of BM, but showed no association with the risk of metastasis to location other than the brain (lung, liver, bone, adrenal gland, and distant lymph nodes) in patients with lung adenocarcinoma. Cox regression analyses showed that patients having the rs6928 GG genotype had a significantly higher risk of BM compared with those with the CC genotype, after adjustment for sex, age, staging, smoking status, surgery, and thoracic radiotherapy (HR: 2.604, P=0.047). Furthermore, patients with the rs6928 CG genotype showed similar higher risk of BM development compared with those with the CC genotype, although the significance was marginal (HR: 1.921, P=0.076). In term of rs5999521, patients having the GG or AG genotype had HRs of 3.001 (P=0.020) and 1.861 (P=0.092), respectively for developing BM compared with those with the AA genotype. In the validation cohort, similar associations between rs6928 and rs5999521 polymorphisms and BM risk were also observed. In the pooled analyses, the association between genetic polymorphisms and risk of BM development became robust. Those who had the rs6928 GG and CG genotypes had 2.033-fold (P=0.033) and 1.910-fold (P=0.012) increased risk of developing BM, respectively, compared with patients with the CC genotype (Figure 1A). For rs5999521, the increased risks of developing BM were 1.993-fold (P=0.037) in patients with the GG genotype and 1.834-fold (P=0.019) in patients with the AG genotype compared with patients with the AA genotype (Table 3) (Figure 1B).
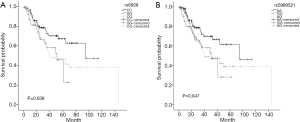
Table 3
| ERK2 | Beijing cohort (n=120) | Changchun cohort (n=213) | Total (n=333) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | HR | 95% CI | P | n | HR | 95% CI | P | n | HR | 95% CI | P | |||
| rs6928 | ||||||||||||||
| CC | 46 | 1.0 (reference) | 76 | 1.0 (reference) | 122 | 1.0 (reference) | ||||||||
| GG | 11 | 2.604 | 1.013–6.696 | 0.047 | 33 | 2.008 | 1.049–5.382 | 0.046 | 44 | 2.033 | 1.057–3.910 | 0.033 | ||
| CG | 63 | 1.921 | 0.934–3.948 | 0.076 | 104 | 2.016 | 0.942–4.315 | 0.071 | 167 | 1.910 | 1.156–3.157 | 0.012 | ||
| rs5999521 | ||||||||||||||
| AA | 12 | 1.0 (reference) | 34 | 1.0 (reference) | 46 | 1.0 (reference) | ||||||||
| GG | 46 | 3.001 | 1.189–7.572 | 0.020 | 73 | 1.906 | 1.194–5.165 | 0.045 | 119 | 1.993 | 1.042–3.810 | 0.037 | ||
| AG | 62 | 1.861 | 0.904–3.832 | 0.092 | 106 | 2.068 | 0.955–4.478 | 0.065 | 168 | 1.834 | 1.106–3.042 | 0.019 | ||
CI, confidence interval; HR, hazard ratio.
EGFR mutation
Data on EGFR mutation status were available in 109 patients. The mutation rates were 38.5% in the discovery cohort and 48.6% in the validation cohort. In multivariate analyses, EGFR mutations were not found to be independent prognostic factors of BM. Otherwise, although the mutation rate was higher in patients with BM, the difference between patients with or without BM was not significant. However, when mutation rate analyses were conducted according to ERK2 polymorphisms, we found that there were differences among patients with specific genotypes. Those with the rs6928 CG genotype, who were shown to have higher risk of BM, had higher EGFR mutation rates compared with those with the CC genotype (P=0.089). In addition, patients having the rs5999521 AG genotype also showed higher EGFR mutation rates than those with the AA genotype (P=0.047; Table 4).
Table 4
| EGFR (n=109) | BM status | rs6928 | rs5999521 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With BM | Without BM | P value | CC | GG | CG | *P value | AA | GG | AG | **P value | |||
| EGFR mutation rate (%) | 47.8 | 44.2 | 0.67 | 40.9 | 33.3 | 53.2 | 0.089 | 40.0 | 33.3 | 54.3 | 0.047 | ||
| Wild-type (n=60) | 12 | 48 | 26 | 12 | 22 | 27 | 12 | 21 | |||||
| Exon 18 mutation (n=2) | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | |||||
| Exon 19 mutation (n=21) | 5 | 16 | 6 | 3 | 12 | 6 | 3 | 12 | |||||
| Exon 21 mutation (n=24) | 4 | 20 | 10 | 3 | 11 | 10 | 3 | 11 | |||||
| Dual mutation (exon19/21) (n=2) | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | |||||
*P value indicates the mutation difference between patients with the rs6928 CG and CC genotypes; **P value indicates the mutation difference between patients with the rs5999521 AG and AA genotypes. BM, brain metastasis; EGFR, epidermal growth factor receptor.
Correlation between genetic variations and other clinical factors in patients with BM
Additional analyses were conducted to explore the correlation between genetic variations and other clinical factors in patients with BM (Table 5). We found that, at time of BM occurrence, patients with genotypes indicating higher risk of BM, tended to have >1 BM lesions. And the difference was significant among rs6928 genotypes (P=0.048) and marginal among rs5999521 genotypes (P=0.064). However, in terms of other clinical factors such as gender, smoking status, age at BM, extracranial metastases, and Karnofsky performance status at BM, no such a correlation was found. The OS differences were not significant among rs6928 or rs5999521 genotypes, either.
Table 5
| Variable | Patients with >1 BMs, N (%) | With extracranial metastases, N (%) | KPS at BM [range] | Gender (male), N (%) | Current smokers, N (%) | Median age at BM [range], yrs |
|---|---|---|---|---|---|---|
| rs6928 | ||||||
| CC (n=25) | 13 (52.0)* | 17 (68.0) | 80 [40–100] | 16 (64.0) | 9 (36.0) | 57 [37–76] |
| GG (n=17) | 10 (58.8) | 8 (47.1) | 90 [60–100] | 10 (58.8) | 6 (35.3) | 61 [25–75] |
| CG (n=55) | 38 (69.1) | 38 (69.1) | 90 [20–100] | 29 (52.7) | 16 (29.1) | 56.5 [38–84] |
| rs5999521 | ||||||
| AA (n=24) | 13 (54.2) | 17 (70.8) | 80 [40–100] | 15 (62.5) | 8 (33.3) | 57.5 [37–76] |
| GG (n=18) | 10 (55.6) | 9 (50.0) | 90 [60–100] | 11 (61.1) | 6 (33.3) | 60.5 [25–75] |
| AG (n=55) | 38 (69.1) | 37 (67.3) | 90 [20–100] | 29 (52.7) | 17 (30.9) | 56.5 [38–84] |
*With statistical significance. BM, brain metastasis; KPS, Karnofsky performance status.
Discussion
In this study, we investigated the association of genetic variations in MAPK with the risk of BM in patients with lung adenocarcinoma. Our results showed that genetic variations in ERK2 rs6928 and rs5999521 were closely associated with the risk of BM. BM is a common complication in patients with NSCLC and should be addressed in clinical practice. Inspired by the successful experience obtained in patients with SCLC, some oncologists have explored the efficacy of PCIs in the NSCLC setting. Unfortunately, survival benefit was not observed in either study (1,12). One likely explanation is that the risk of BM was heterogeneous across patients with NSCLC. Hence, patients with NSCLC should be stratified according to the risk of BM. Only those at risk of BM should be candidates for BM prevention.
In order to simplify the issue, we confined our study to patients with adenocarcinoma. Eligibility was not only based on the higher incidence of BM in adenocarcinoma compared with other histologies (3,14) but also on the frequency of EGFR mutations, which have been shown to be associated with BM in several studies. SNPs have been widely used in the study of cancer susceptibility. Some researchers have employed SNPs to explore the risk of BM (16,20). MAPKs are key regulators of cell growth and survival in physiological and pathological processes (21,22). Aberrant MAPK signalling plays critical roles in the development and progression of human cancers, including NSCLC (23,24). Thus, germline polymorphisms may be used to identify patients at risk of metastasis. In addition, MAPK has been shown to be an important mediator of EGFR/MET crosstalk, which is essential for the metastatic behaviours of NSCLC cells observed in vivo and in vitro. As shown in our study, ERK2 rs6928 and rs5999521 were closely associated with the risk of BM after adjustment. These correlations were organ specific since they were not established with other metastatic sites. Furthermore, the results were consistent between the discovery and validation cohorts and became robust in pooled analysis. Rs6928, located in 22q11.2, is a genetic downstream transcript variant. Some studies have shown that rs6928 is correlated with treatment response in patients with depression (25). The polymorphism rs5999521, an intronic variant, is associated with risk of polycystic ovarian syndrome (26). However, the underlying mechanisms through which these polymorphisms contribute to BM are still unclear.
EGFR mutation status is closely associated with the risk of BM in patients with NSCLC, particularly when conventional chemotherapy applied (17,18). Patients with EGFR mutations have a significantly higher risk of BM compared with those with wild-type EGFR. Unfortunately, in our series, this was not validated in multivariate analysis. In addition to the small population of patients with available EGFR mutation status data, we also attribute these findings to application of tyrosine kinase inhibitors (TKIs) (only three patients with EGFR mutations received conventional chemotherapy before the occurrence of BM in our series) because TKIs can reduce the risk of BM development (27,28). In order to further explore the correlations between MAPK polymorphisms and EGFR mutation status, we analysed EGFR mutation rates according to patient genotype. As a result, in patients with the rs6928 CG genotype, who showed a higher risk of BM development compare with those with the CC genotype, the EGFR mutation rate was also higher than that of patients with the CC genotype. Similarly, the EGFR mutation rate in patients with the rs5999521 AG genotype was higher than that in patients with the AA genotype. These results suggested that EGFR mutations may contribute to the increased risk of BM in patients with specific phenotypes, whereas alternative mechanisms may be involved in patients with other phenotypes.
In addition to the value of BM risk evaluation, we also found correlations between SNPs and clinical factors at the time of BM occurrence. It was indicated that, those who had genotypes of higher risk of BM, tended to have multiple BM lesions. As it was validated in many previous studies, number of BM lesions, extracranial metastases, age and KPS status, are all independent factors of survival in patients with BM. In the current study, probably due to smaller samples, marginal survival significance was found among patients with different number of BM lesions (1 vs. 2–3 vs. >3, P=0.067). Hence, in patients with BM, survival analyses did not found difference among genotypes, either in rs6928 or rs5999521. Even analyses were conducted in the whole series, the curves did not separate significantly. That means, those SNPs identified in the current study may not powerful enough to predict OS in this population.
Conclusions
In this study, we found that ERK2 rs6928 and rs5999521 SNPs were associated with the risk of BM in patients with lung adenocarcinoma. Furthermore, EGFR mutations may contribute to the increased risk of BM in patients with specific genotypes of rs6928 or rs5999521. Thus, these SNPs may be potential biomarkers, in combination with clinicopathological variables, for identification of patients at high risk of BM who would be candidates for studies of BM prevention.
Acknowledgments
The manuscript was edited by Elsevier Language Editing and American Journal Experts to meet the language standards of the journal.
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-3069
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tcr-20-3069
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-3069). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review boards and ethics committees of Beijing Tiantan Hospital of Capital Medical University (KYSB2010-170-01) and The First Hospital of Jilin University (2011-031) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pöttgen C, Eberhardt W, Grannass A, et al. Prophylactic cranial irradiation in operable stage IIIA non small-cell lung cancer treated with neoadjuvant chemoradiotherapy: results from a German multicenter randomized trial. J Clin Oncol 2007;25:4987-92. [Crossref] [PubMed]
- Gore EM, Bae K, Wong SJ, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol 2011;29:272-8. [Crossref] [PubMed]
- Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: risk factors analysis. Cancer 2002;95:605-12. [Crossref] [PubMed]
- Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510-4. [Crossref] [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Zabel A, Debus J. Treatment of brain metastases from non-small-cell lung cancer (NSCLC): radiotherapy. Lung Cancer 2004;45:S247-52. [Crossref] [PubMed]
- Davey P, Hoegler D, Ennis M, et al. A phase III study of accelerated versus conventional hypofractionated whole brain irradiation in patients of good performance status with brain metastases not suitable for surgical excision. Radiother Oncol 2008;88:173-6. [Crossref] [PubMed]
- Meert AP, Paesmans M, Berghmans T, et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer 2001;1:5. [Crossref] [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [Crossref] [PubMed]
- Putora PM, Glatzer M, Belderbos J, et al. Prophylactic cranial irradiation in stage IV small cell lung cancer: Selection of patients amongst European IASLC and ESTRO experts. Radiother Oncol 2019;133:163-6. [Crossref] [PubMed]
- Kim TG, Pyo H, Ahn YC, et al. Role of prophylactic cranial irradiation for elderly patients with limited-disease small-cell lung cancer: inverse probability of treatment weighting using propensity score. J Radiat Res 2019;60:630-8. [Crossref] [PubMed]
- Sun A, Hu C, Wong SJ, et al. Prophylactic Cranial Irradiation vs Observation in Patients With Locally Advanced Non-Small Cell Lung Cancer: A Long-term Update of the NRG Oncology/RTOG 0214 Phase 3 Randomized Clinical Trial. JAMA Oncol 2019;5:847-55. [Crossref] [PubMed]
- Robnett TJ, Machtay M, Stevenson JP, et al. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol 2001;19:1344-9. [Crossref] [PubMed]
- Wang SY, Ye X, Ou W, et al. Risk of cerebral metastases for postoperative locally advanced non-small-cell lung cancer. Lung Cancer 2009;64:238-43. [Crossref] [PubMed]
- Li Q, Wu H, Chen B, et al. SNPs in the TGF-beta signaling pathway are associated with increased risk of brain metastasis in patients with non-small-cell lung cancer. PLoS One 2012;7:e51713. [Crossref] [PubMed]
- Li Q, Yang J, Yu Q, et al. Associations between single-nucleotide polymorphisms in the PI3K-PTEN-AKT-mTOR pathway and increased risk of brain metastasis in patients with non-small cell lung cancer. Clin Cancer Res 2013;19:6252-60. [Crossref] [PubMed]
- Li B, Sun SZ, Yang M, et al. The correlation between EGFR mutation status and the risk of brain metastasis in patients with lung adenocarcinoma. J Neurooncol 2015;124:79-85. [Crossref] [PubMed]
- Qin Q, Peng B, Li B. The impact of epidermal growth factor receptor mutations on the efficacy of definitive chemoradiotherapy in patients with locally advanced unresectable stage III non-small cell lung cancer: a systematic review and meta-analysis. Expert Rev Anticancer Ther 2019;19:533-9. [Crossref] [PubMed]
- Breindel JL, Haskins JW, Cowell EP, et al. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res 2013;73:5053-65. [Crossref] [PubMed]
- Le Rhun E, Bertrand N, Dumont A, et al. Identification of single nucleotide polymorphisms of the PI3K-AKT-mTOR pathway as a risk factor of central nervous system metastasis in metastatic breast cancer. Eur J Cancer 2017;87:189-98. [Crossref] [PubMed]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 2009;9:537-49. [Crossref] [PubMed]
- Zhang YL, Dong C. MAP kinases in immune responses. Cell Mol Immunol 2005;2:20-7. [PubMed]
- Yue SJ, Zhang PX, Zhu Y, et al. A Ferulic Acid Derivative FXS-3 Inhibits Proliferation and Metastasis of Human Lung Cancer A549 Cells via Positive JNK Signaling Pathway and Negative ERK/p38, AKT/mTOR and MEK/ERK Signaling Pathways. Molecules 2019;24:2165. [Crossref] [PubMed]
- Yang Y, Zhao J, Hao D, et al. Increased SPK1 expression promotes cell growth by activating the ERK1/2 signaling in non-small-cell lung cancer. Anticancer Drugs 2019;30:458-65. [Crossref] [PubMed]
- Fabbri C, Crisafulli C, Calati R, et al. Neuroplasticity and second messenger pathways in antidepressant efficacy: pharmacogenetic results from a prospective trial investigating treatment resistance. Eur Arch Psychiatry Clin Neurosci 2017;267:723-35. [Crossref] [PubMed]
- Hu L, Zhang Y, Chen L, et al. MAPK and ERK polymorphisms are associated with PCOS risk in Chinese women. Oncotarget 2017;8:100261-8. [Crossref] [PubMed]
- Su PL, Wu YL, Chang WY, et al. Preventing and treating brain metastases with three first-line EGFR-tyrosine kinase inhibitors in patients with EGFR mutation-positive advanced non-small cell lung cancer. Ther Adv Med Oncol 2018;10:1758835918797589. [Crossref] [PubMed]
- Zhao X, Zhu G, Chen H, et al. Efficacy of icotinib versus traditional chemotherapy as first-line treatment for preventing brain metastasis from advanced lung adenocarcinoma in patients with epidermal growth factor receptor-sensitive mutation. J Cancer Res Ther 2014;10:C155-9. [Crossref] [PubMed]


