
RCHOP-14 therapy versus RCHOP-21 therapy for people with aggressive or advanced-stage indolent B-cell non-Hodgkins lymphoma: a systematic review and meta-analysis
Introduction
Aggressive and indolent lymphomas are two subtypes of B-cell-derived non-Hodgkins lymphoma (NHL) and are treated with different chemotherapy regimens depending on the prognosis. Aggressive NHL is a highly aggressive malignancy with a poor outcome. It is a greatly chemo-sensitive tumor and is highly curable (1). An advanced-stage indolent NHL is often incurable and can easily transform into an aggressive lymphoma. However, it can be alleviated with a regimen of rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone (RCHOP) (2,3). Diffuse large B-cell lymphoma (DLBCL), a disease of biologically, histopathologically, and clinically heterogeneous entities (4), is the most common subtype of aggressive lymphoma. The median survival time of patients with DLBCL who did not undergo prompt treatment is less than 1 year on account of the DLBCL’s aggressive nature (5,6). For a long time, the first-line chemotherapy treatment for DLBCL was cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) treatment. It is more reasonable to choose to combine this treatment with CHOP-21 every 3 weeks. Several randomized controlled clinical trials (RCTs) regarding the survival analysis of dose-intensified regimens were conducted, which showed that the CHOP-14 2-week cycle of chemotherapy is superior to the CHOP-21 treatment (7,8).
The human/murine chimeric anti-CD20 monoclonal antibody called rituximab has a credible efficacy. It is well-defined and adequately safe for patients with various CD20-expressing lymphoid malignancies, such as aggressive and indolent forms of B-cell NHL (9). Follicular lymphoma (FL) is a neoplasm comprising germinal center B cells and is a subgroup of indolent lymphomas. The standard option for patients with advanced-stage FL is rituximab-CHOP (10). NHL (PMBL) is a unique subtype of DLBCL originating from thymic B-cells in the mediastinum. The RCHOP regimen, with or without consolidative radiotherapy, is first-line PMBL management (11). It can be observed in all of these diseases, which include DLBL, FL, mantle cell lymphoma, and chronic lymphocytic leukemia, that rituximab-based treatment not only extends the time of the patient’s progression-free survival (PFS) but also prolongs his/her overall survival (OS) time (12). Therefore, it is meaningful to discuss the choice between rituximab-based RCHOP-14 and 21 chemotherapy regimens for aggressive or advanced-stage indolent B-cell NHL.
We implement trails from RCTs and observational comparative studies (OCSs) to estimate the efficacy and toxicity of a chemotherapy regimen, comparing RCHOP-14 to RCHOP-21 in patients with B-cell NHL. The results include complete response (CR), PFS, OS, and toxicity levels. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tcr-20-3123).
Methods
Search strategy
First, we conducted a systematic and comprehensive search from original to January 2020 throughout databases, including PubMed, Web of Science, Embase, Cochrane Library, and ClinicalTrials.gov. The predefined keywords were used with Boolean operators for the search: “RCHOP-14 AND RCHOP-21” OR “dose-dense” AND “lymphoma”. The electronic search was complemented by a manual search for additional articles in reference lists and previous reviews, rendering a full-scale investigation.
Selection criteria
We enrolled trials meeting the below inclusion criteria: (I) high-quality OCS and studies based on RCTs; (II) participants newly diagnosed with aggressive lymphoma at clinical stages I–IV or untreated advanced-stage indolent B-cell NHL; (III) comparative analysis of RCHOP-14 and RCHOP-21 for treating B-cell NHL; (IV) follow-up duration longer than 36 months (V) an existence outcome of CR, PFS or OS in the articles. Duplicated data that might lead to an overestimation of intervention effects was contained cautiously. Review articles, conference abstracts, nonhuman studies, case reports, abstracts, and unpublished data were excluded from consideration. Moreover, studies that did not exclude data were not included. If there were differences regarding which studies should be included, experts decided whether to include them or not.
Data extraction and quality assessment
Relevant Data were independently extracted from included articles by two authors. The following data were extracted: the first author, published year, location, disease, stage, median age, median follow-up, number of patients with international prognostic index (IPI) at different levels, sample size, number of cycles, and clinical outcomes (including CR, PFS, OS and toxicity). We assessed the quality of RCTs using the Cochrane Collaboration’s risk of bias tool, Rev Man 5.3. The quality of selected studies was appraised with methodological domains as follows: risks of selection, performance, detection, attrition, and reporting biases. For the included study, types of bias are divided into three levels: low, unclear, high. The Newcastle-Ottawa scale (NOS) uses three categories, the selection of study groups, comparability, and outcome assessment, respectively, to evaluate the risk of OCS biases.
Statistical analysis
A meta-analysis of variables with three or more studies was performed when the outcome was reported. Statistical heterogeneity among individual studies was calculated by the P and I2 test, where heterogeneity will be considered substantive if I2> 50% (13). The fixed-effect model and the random-effects models were utilized for both consistent and heterogeneous studies in accordance with the previously published guidelines for statistical reporting and a systematic review manual on Cochrane interventions. PFS and OS, as the dichotomous data, were reported with hazard ratios (HRs) and 95% CIs. HR is calculated by the inverse of variance. It is used to weigh the size of the individual effect. The CR rate was calculated through the odds ratio (OR) with the random-effects model (M-H methods) and adverse events (AEs), used to analyze the risk ratio (RR), were calculated with the same model. Next, the forest map for meta-analysis was drawn. When possible, sensibility analysis was conducted to investigate the origins of heterogeneity. Funnel plots were performed to attest the presence of publication bias. All statistical analyses were conducted in Review Manager 5.3.
Results
Description of studies
A total of 403 potentially relevant studies were ascertained after the initial search (Figure 1). Of these, 31 articles were from PubMed, 59 from Embase, 173 from Web of Science, 134 from Cochrane Library and 6 from clinicaltrials.gov. One hundred eighty-one irrelevant articles and 142 duplicated articles were expurgated by carefully reviewing the titles and abstracts. Sixty-six pieces of literature were deleted for the reason that these trials were conference reports, non-original or scarce data, review or meta-analysis, or weren’t RCHOP-14 vs. RCHOP-21 and related results. Finally, six RCTs—Cunningham et al. (2013), Delarue et al. (2013), Payandeh et al. (2016), Watanabe et al. (2018), Li et al., (2019), and Gleeson et al. (2016)—and two OCSs—Wästerlid et al. (2017) and Knauf et al. (2019)—met all inclusion criteria entered in this meta-analysis (14-21).
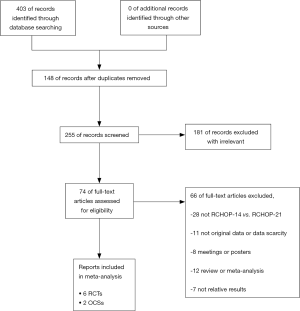
Patients types
In total, the five studies included 5,565 patients with B-cell NHL, of whom 2,892 underwent RCHOP-14 and 2,673 only underwent RCHOP-21. The experimental characteristics of each RCT are summarized in Table 1. Most of the enrolled trials were from different countries, four of which are in Europe. Four trails accounting for studies were from Asia. We collected patients above the age of 18 with clinical stage I–IV aggressive lymphoma and untreated stage III–IVV indolent B-cell NHL. The granulocyte colony-stimulating factor (G-CSF) was applied to both the RCHOP-14 group and the RCHOP-21 group to shorten the CHOP treatment. Stimulating Moreover, the sample sizes for individual studies varied widely from 50 to 2,106 despite being multi-center clinical trials.
Table 1
| Study | Location | Disease | Stage | Median follow-up (months) | Sample size | Number of cycles | Use of G-CSF | |||
|---|---|---|---|---|---|---|---|---|---|---|
| RCHOP-14/RCHOP-21 | RCHOP-14/RCHOP-21 | RCHOP-14 | RCHOP-21 | |||||||
| Cunningham 2013 (14) | UK | DLBCL | I–IV | 46 | 540/540 | 6 plus 2 R/8 | Given to all patients | 54% of patients | ||
| Delarue 2013 (15) | France, Belgium, Switzer, Portugal | DLBCL | I–IV | 56 | 304/298 | 8/8 | 90% of patients, decision of the treating physician | 74% of patients, decision of the treating physician | ||
| Gleeson 2016 (21) | UK | DLBCL | I–II | 86.4 | 22/28 | 6 plus 2 R/8 | Given to all patients | 54% of patients | ||
| Payandeh 2016 (17) | Iran | B-cell NHL | III–IV | 45 | 66/77 | 6–8/6–8 | Given to all patients | At the desertion of the treating physician | ||
| Watanabe 2018 (16) | Japan | Untreated advanced-stage FL | III–IV | 134.4 | 151/149 | 6/6 | Given to all patients | At the desertion of the treating physician | ||
| Li 2019 (18) | China | DLBCL | I–IV | 45.6 | 349/353 | 6–8/6–8 | Given to all patients | The investigator’s discretion | ||
| Wästerlid 2017 (20)* | Swedish | PMBL | I–IV | 47.4 | 1196/910 | 6/6 | Not report | Not report | ||
| Knauf 2019 (19)* | German | DLBCL | I–IV | 60 | 264/318 | 6/6 | 73% of patients use it at least once | 48.7% of patients use it at least once | ||
*, OCSs. G-CSF, granulocyte colony-stimulating factor; RCHOP, rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone; DLBCL, diffuse large B-cell lymphoma; PMBL, primary mediastinal B-cell lymphoma; FL, follicular lymphoma; R, rituximab; OCSs, observational comparative studies.
Quality assessment
Six RCTs were assessed as low risk in the light of a suitable option (Figure 2A,B). However, four RCTs had a high risk of selection bias as allocation concealment (14,16,18,21). All funnel plots of PFS and OS were symmetrical, indicating no publication bias (Figure 2C,D). The selection of high-quality OCSs was based on a validated tool. Two OCSs were evaluated by NOS (Table 2), and the results suggested that both of them were high-quality literature.

Table 2
| Study | Items | Wästerlid 2017 (20) | Knauf 2019 (19) |
|---|---|---|---|
| Selection | Representativeness of the exposed cohort | * | * |
| Selection of the non-exposed cohort | * | * | |
| Ascertainment of exposure | * | * | |
| Demonstration that outcome of interest was not present at start of study | * | * | |
| Comparability | Comparability of cohorts on the basis of the design or analysis | ** | ** |
| Outcome | Assessment of outcome | * | * |
| Was follow-up long enough for outcomes to occur | * | * | |
| Adequacy of follow up of cohorts | * | * |
*, star-rating in NOS, each study can have a maximum of one star per entry in “Selection”, “Outcome” and a maximum of two stars per entry in “Comparability”. OCSs, observational comparative studies; NOS, Newcastle-Ottawa scale.
Efficacy
CR rate data were available from eight studies (14-21), incorporating 2,657 patients from the RCHOP-14 therapeutic regimen and 2,415 patients from the RCHOP-21 regimen. A significant heterogeneity was found within these two regimes (χ2=17.69, P=0.007, I2=66%) (Figure 3). The random-effects model was subsequently used. The CR rate did not meliorate with RCHOP-14 regimens in patients (OR =0.96, 95% CI: 0.76–1.23, P=0.76). The results of the RCTs and OCSs were consistent, so we calculated the data together and displayed it on a graph.
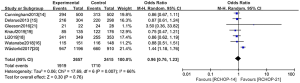
Survival
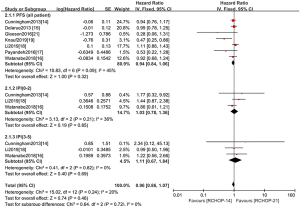
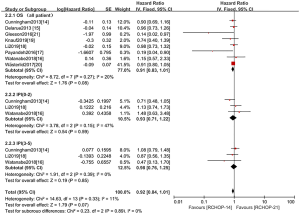
The PFS and OS of RCHOP-14 vs. RCHOP-21 was the main long-term clinical outcome evaluation with B-cell lymphoma. Figures 4,5 suggest that no significant between-trial heterogeneity was observed between PFS and OS. Hence, we chose the fixed-effect model. The results of the OCSs were consistent with the RCTs, so we presented these data in a single graph and stratified the clinical outcomes of patients with different prognoses based on the IPI scores. For the comparison, PFS was curtailed in RCHOP-14, but it showed no significant difference (HR =0.94, 95% CI: 0.84–1.06, P=0.32). Results were not altered after differentiating patients with different IPI scores (Figure 4). Regarding OS, RCHOP-14 was superior to RCHOP-21 (HR =0.91, 95% CI: 0.83–1.01, P=0.08) (Figure 5). However, there was still no statistical difference among the trials. After stratification according to the IPI score, the OS of patients with different prognoses was in agreement with the outcome indicators of all patients.
Treatment-related toxicity
AEs, including both hematological and non-hematological toxicities, with both RCHOP-14 and RCHOP-21 treatment protocols were reviewed in all RCTs. Table 3 summarizes the grade ≥3 AEs. We have used RR values to compare the AEs of the five studies in the supplementary picture, and the toxicity of the RCHOP-14 regimen and RCHOP-21 regimen does not have a significantly high risk (RR =0.98, 95% CI: 0.83–1.15, P=0.73). I2=85% suggested greater heterogeneity among the trials, which was statistically significant. The subgroup analysis results on hematological AEs show that the incidences of thrombocytopenia (RR =0.87, 95% CI: 0.60–1.25, P=0.44) were higher in the RCHOP-14 arm (9,10,12-14), although this has no statistical significance. One of the subgroup analyses with patients who received RCHOP-21, who have a higher trend of anemia when ceasing treatment Watanabe et al. (RR =1.15, 95% CI: 0.88–1.50, P=0.29), was observed (14,17,18). The subgroup analysis on non-hematological AEs indicates that patients treated with RCHOP-21 had a higher risk of neurological-related, which was not statistically significant (RR =1.41, 95% CI: 0.85–2.33, P=0.18).
Table 3
| Specific AEs | Number of studies | RCHOP-14 | RCHOP-21 | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pts with SAE/total pts | Pts with SAE/total pts | Relative risk (95% CI) | P value | P value | I2 (%) | ||||
| Neutropenia | 5 | 722/1,340 | 896/1,408 | 0.93 (0.64–1.36) | 0.71 | <0.00001 | 98 | ||
| Thrombocytopenia | 5 | 102/1,340 | 132/1,408 | 0.87 (0.60–1.25) | 0.44 | 0.15 | 41 | ||
| Anemia | 4 | 121/770 | 97/874 | 1.15 (0.88–1.50) | 0.29 | 0.48 | 0 | ||
| Febrile neutropenia | 3 | 103/989 | 134/978 | 0.66 (0.33–1.30) | 0.23 | 0.001 | 85 | ||
| Infection | 4 | 209/1,238 | 225/1,331 | 1.18 (0.72–1.91) | 0.51 | 0.0003 | 84 | ||
| Gastrointestinal toxicity | 4 | 70/1,238 | 74/1,331 | 1.00 (0.73–1.38) | 0.98 | 0.52 | 0 | ||
| Increase in amount of liver enzymes | 3 | 21/521 | 21/521 | 1.04 (0.58–1.86) | 0.9 | 0.99 | 0 | ||
| Cardiac-related | 3 | 15/521 | 14/521 | 1.04 (0.15–7.34) | 0.97 | 0.02 | 74 | ||
| Neurological-related | 3 | 80/989 | 57/978 | 1.41 (0.85–2.33) | 0.18 | 0.19 | 40 | ||
SAE, severe adverse event; AE, adverse event; RCHOP, rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone.
Discussion
At a clinical level, RCHOP-14 and RCHOP-21 are the two different international standards used for the treatment of B-cell lymphoma. This manuscript implies that the CR rate, PFS, and OS were higher in patients who were assigned RCHOP-14 therapy. However, its outcomes did not differ significantly. This indicates that improvement of the CR rate, PFS, and OS in these patients may not be possible through RCHOP-14. More RCTs are required in order to confirm whether the addition of radiotherapy can change this outcome or not. The previous meta-analysis shows that the treatment options of RCHOP were manifested to prolong OS when given every 14 days instead of 21 days in case rituximab was omitted (22). Our analysis showed that RCHOP-14 and RCHOP-21 have no statistically significant difference in PFS and OS.
Toxicity was an important endpoint of our study. There is a higher risk of infectious complications associated with RCHOP14, particularly febrile neutropenia, due to infections caused by opportunistic pathogens (23-25). However, our study shows that the toxicity of RCHOP-14 regimen is the same as the toxicity of the RCHOP-21 regimen in B-cell patients, rather than higher. One reason why the RCHOP-14 regimen has the same safety-rate as the prophylactic recombinant human G-CSF. G-CSF has often been used to potentiate the antibody-dependent cell-mediated cytotoxicity of rituximab (26,27), after which CHOP intervals can be shortened (7,8,28,29). More patients who were given the prophylactic recombinant human G-CSF every 14 days developed grade 3–4 neutropenia than reported previously (14). One type of toxicity is thrombocytopenia, especially in the RCHOP-14 regimen. It may increase the chance of intravenous platelet. Meanwhile, anemia is more likely to occur for RCHOP-21, which leads frequent transfusions. Another apparent reason is that there is greater heterogeneity between subgroups and the results may be unreliable.
As far as we can see, this study is the first meta-analysis to assess the efficacy and toxicity of the CHOP regimen in patients with aggressive or advanced-stage indolent B-cell NHL based on rituximab. It is also the first to analyze survival outcomes for patients with different prognostic outcomes based on IPI scores. The data suggests that we could face type 2 errors in the RCTs. The main argument for including OCSs is trying to avoid making this mistake. However, the meta-analysis still has some limitations. Firstly, it is possible that two studies caused performance and detection biases because of they were open-label trails. In the second place, the low number of included studies made it difficult for a detailed, in-depth probe and an interpretation of a potential underlying heterogeneity. When ascertaining heterogeneity among individual studies for toxicity, which is still significantly high after removing the relevant study. The reason for the high heterogeneity may be the different B-cell NHL prognoses and the inconsistent chemotherapy cycle. Therefore, we need more RCTs to explore the potential causes of heterogeneity. In the end, other covariates, such as supportive therapy, preventive measures of toxicity, and the proficiency of a doctor, could not be balanced in the study.
Conclusions
To sum up the study, an analysis of data from clinical trials of RCHOP-14 treatment showed that the therapies are safe and effective compared with the RCHOP-21 treatment. However, there was no significant difference in PFS and OS, and that it produces clinical responses similar to those in CR rate. Additional considerations in regard to choosing the treatment strategy and balancing treatment-related toxicity may help us to decide whether to treat with RCHOP-14 or RCHOP-21.
Acknowledgments
The article has been proofed by professor Cole in Proofed Inc.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tcr-20-3123
Peer Review File: Available at http://dx.doi.org/10.21037/tcr-20-3123
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-20-3123). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mugnaini EN, Ghosh N. Lymphoma. Prim Care 2016;43:661-75. [Crossref] [PubMed]
- Mondello P, Steiner N, Willenbacher W, et al. Bendamustine plus rituximab versus R-CHOP as first-line treatment for patients with indolent non-Hodgkin's lymphoma: evidence from a multicenter, retrospective study. Ann Hematol 2016;95:1107-14. [Crossref] [PubMed]
- Igarashi T, Ogura M, Itoh K, et al. Japanese phase II study of rituximab maintenance for untreated indolent B-cell non-Hodgkin lymphoma with high tumor burden. Int J Hematol 2016;104:700-8. [Crossref] [PubMed]
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503-11. [Crossref] [PubMed]
- Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin 2010;60:393-408. [Crossref] [PubMed]
- Sinha R, Nastoupil L, Flowers CR. Treatment strategies for patients with diffuse large B-cell lymphoma: past, present, and future. Blood Lymphat Cancer 2012;2012:87-98. [PubMed]
- Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 2004;104:634-41. [Crossref] [PubMed]
- Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood 2004;104:626-33. [Crossref] [PubMed]
- Salles G, Barrett M, Foa R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther 2017;34:2232-73. [Crossref] [PubMed]
- Ando K. Follicular lymphoma: recent advances. Rinsho Ketsueki 2018;59:2104-8. [PubMed]
- Soumerai JD, Hellmann MD, Feng Y, et al. Treatment of primary mediastinal B-cell lymphoma with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone is associated with a high rate of primary refractory disease. Leuk Lymphoma 2014;55:538-43. [Crossref] [PubMed]
- Economopoulos T, Psyrri A, Dimopoulos MA, et al. CEOP-21 versus CEOP-14 chemotherapy with or without rituximab for the first-line treatment of patients with aggressive lymphomas: results of the HE22A99 trial of the Hellenic Cooperative Oncology Group. Cancer J 2007;13:327-34. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 2013;381:1817-26. [Crossref] [PubMed]
- Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013;14:525-33. [Crossref] [PubMed]
- Watanabe T, Tobinai K, Wakabayashi M, et al. Outcomes after R-CHOP in patients with newly diagnosed advanced follicular lymphoma: a 10-year follow-up analysis of the JCOG0203 trial. Lancet Haematol 2018;5:e520-31. [Crossref] [PubMed]
- Payandeh M, Najafi S, Shojaiyan FZ, et al. Phase III of study of R-CHOP-21 vs R-CHOP-14 for untreated stage III and IV B-cell non-Hodgkin's lymphoma: a report from Iran. Asian Pac J Cancer Prev 2016;17:1513-7. [Crossref] [PubMed]
- Li X, Huang H, Xu B, et al. Dose-dense rituximab-CHOP versus standard rituximab-CHOP in newly diagnosed Chinese patients with diffuse large B-cell lymphoma: a randomized, multicenter, open-label phase 3 trial. Cancer Res Treat 2019;51:919-32. [Crossref] [PubMed]
- Knauf W, Abenhardt W, Mohm J, et al. Similar effectiveness of R-CHOP-14 and -21 in diffuse large B-cell lymphoma-data from the prospective German Tumour Registry Lymphatic Neoplasms. Eur J Haematol 2019;103:460-71. [Crossref] [PubMed]
- Wästerlid T, Hartman L, Székely E, et al. Impact on survival of addition of etoposide to primary chemotherapy in diffuse large B-cell lymphoma: a Swedish Lymphoma Registry study. Hematol Oncol 2017;35:151-7. [Crossref] [PubMed]
- Gleeson M, Hawkes EA, Cunningham D, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) in the management of primary mediastinal B-cell lymphoma: a subgroup analysis of the UK NCRI R-CHOP 14 versus 21 trial. Br J Haematol 2016;175:668-72. [Crossref] [PubMed]
- Vidal L, Shpilberg O, Gurion R, et al. CHOP-like-14 compared to CHOP-like-21 for patients with aggressive lymphoma--a meta-analysis of randomized controlled trials. Acta Oncol 2016;55:77-84. [Crossref] [PubMed]
- Brusamolino E, Rusconi C, Montalbetti L, et al. Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: a phase II study of feasibility and toxicity. Haematologica 2006;91:496-502. [PubMed]
- Kamel S, O'Connor S, Lee N, et al. High incidence of Pneumocystis jirovecii pneumonia in patients receiving biweekly rituximab and cyclophosphamide, adriamycin, vincristine, and prednisone. Leuk Lymphoma 2010;51:797-801. [Crossref] [PubMed]
- Tadmor T, McLaughlin P, Polliack A. A resurgence of Pneumocystis in aggressive lymphoma treated with R-CHOP-14: the price of a dose-dense regimen? Leuk Lymphoma 2010;51:737-8. [Crossref] [PubMed]
- Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, et al. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin's lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res 2003;9:5866-73. [PubMed]
- Cartron G, Zhao-Yang L, Baudard M, et al. Granulocyte-macrophage colony-stimulating factor potentiates rituximab in patients with relapsed follicular lymphoma: results of a phase II study. J Clin Oncol 2008;26:2725-31. [Crossref] [PubMed]
- Ohmachi K, Tobinai K, Kobayashi Y, et al. Phase III trial of CHOP-21 versus CHOP-14 for aggressive non-Hodgkin's lymphoma: final results of the Japan Clinical Oncology Group Study, JCOG 9809. Ann Oncol 2011;22:1382-91. [Crossref] [PubMed]
- Itoh K, Ohtsu T, Fukuda H, et al. Randomized phase II study of biweekly CHOP and dose-escalated CHOP with prophylactic use of lenograstim (glycosylated G-CSF) in aggressive non-Hodgkin's lymphoma: Japan Clinical Oncology Group Study 9505. Ann Oncol 2002;13:1347-55. [Crossref] [PubMed]


