
Lysophosphatidic acid in prostate cancer progression
Introduction
Prostate cancer is one of the most common cancers diagnosed and the second leading cause of deaths due to cancer in American men. According to the estimation from the American Cancer Society in 2015, approximately 1 in 7 men will be diagnosed with prostate cancer during their lifetime. In men aged over 65, approximately 6 cases in 10 are diagnosed while one in 38 men dies of prostate cancer. Most prostate cancers developed from gland cells and are therefore classified as prostate adenocarcinoma. The growth rate of most prostate cancer is slow, although some are highly malignant and grow and spread quickly. The male hormone androgen is known to promote prostate cancer growth and survival through activation of androgen receptors (AR) (1-4). Therefore, hormone therapy has been used clinically to inhibit prostate cancer growth and spread via androgen deprivation or blockade. However, prostate cancer cells tend to resist this treatment and transform into more aggressive and highly metastatic androgen-independent cells. Unfortunately, the details of this transformation have not been clarified. Nowadays, researchers are trying to find a potential treatment for advanced prostate cancer patients by understanding the detailed molecular mechanisms underpinning androgen-independent prostate cancer cells. Lysophosphatidic acid (LPA) is a simple phospholipid involved in multiple cellular events in almost mammalian cell types. It has been known that LPA binds to LPA receptors and subsequently activates intracellular signaling pathway to regulate prostate cancer cell proliferation (5), survival (6), invasion (7) and migration (8). These functions are dependent on the expression of LPA receptors and activation of downstream signaling transduction pathways. This suggests that LPA receptors are critical for prostate cancer progression. Here, we review the functions of LPA as well as its receptors in prostate cancer progression and how LPA signals mediate cellular functions in prostate cancer cells. In addition, our study shows that LPA stimulates the expressions of VEGF-C in prostate cancer cells (9). These effects are mediated through LPA receptors and ROS production (9,10). Our results, therefore, suggest that blocking LPA signals via targeting LPA receptors and downstream effectors may prevent lymphangiogenesis as well as lymphatic metastasis in advanced prostate cancer. The pathological significances of LPA in different prostate cancer cells are summarized in Table 1.
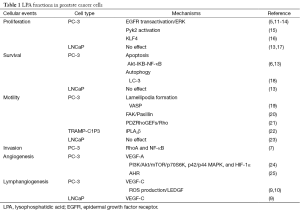
Full table
LPA functions in prostate cancer cell proliferation and survival
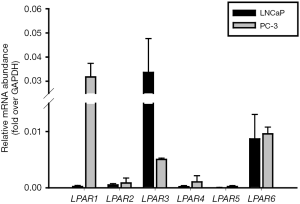
The first study of LPA in prostate cancer was conducted by Qi in 1998. It showed that LPA stimulates cell proliferation of human androgen-insensitive prostate cancer PC-3 cells (5). This mitogenic effect is through phosphorylation of extracellular-signal-regulated kinases (ERKs) (11-13). The upstream regulator has been illustrated that epidermal growth factor receptors (EGFRs) are activated in response to LPA. Similar to the effect of LPA in the fibroblasts, LPA has found to trigger an outside-in signal through LPA receptors, and then inside-out activation of matrix metalloproteinase (MMP) to cleavage extracellular EGF-like ligands. However, this transactivation was not mediated by the shedding of heparin-binding EGF (14,26). Modified EGF-like ligands stimulate the outside-in signaling again via phosphorylate EGFRs and activate downstream intracellular ERKs in prostate cancer cells (14). Conversely, androgen-dependent LNCaP cells do not respond to LPA in terms of cell growth or ERK phosphorylation (13,17). The expression profile of LPA receptors in both cell lines has shown by Guo in 2006 and Figure 1; this may explain the differences between two cell types (17). Not only ERK activation but also other intracellular factors may be involved in LPA-mediated prostate cancer growth. A cytoplasmic protein tyrosine kinase, proline-rich tyrosine kinase 2 (Pyk2), expresses in prostate epithelium and responded to LPA (15). LPA activates Pyk2 in PC-3 cells. Phosphorylated Pyk2 acts as an upstream mediator of the Jun amino-terminal kinase (JNK) signaling pathway (27) and stimulates the Ras-MAPK signaling pathway (28), promoting prostate cancer cell proliferation in vitro. Another report showed that one of Yamanaka factors krüppel-like factor 4 (KLF4) mediates LPA-stimulated proliferation and migration of highly metastatic PC-3 (PC-3M) cells (29). KLF4 is a zinc finger DNA-binding protein regulating proliferation, differentiation, apoptosis, as well as somatic cell reprogramming. LPA induces KLF4 mRNA expression which could further promote prostate cancer cell proliferation. Intriguingly, other polyunstaturated fatty acid may also affect LPA signaling as well as cell proliferation. Omega-3 fatty acids (n-3 Fas) and free fatty acid receptor 4 (FFAR4) agonists inhibit LPA-induced proliferation in moderate metastatic DU-145 cells by blocking ERK, FAK, and p70S6K. Likewise, expression of the matricellular protein CCN1 in response to LPA is regulated by n-3 Fas (16). Together, these reports suggested that blocking LPA signaling and downstream effectors may inhibit prostate cancer growth especially highly malignant cancer cells.
LPA also mediates survival of prostate cancer cells. LPA inhibits serum deprivation-induced apoptosis of PC-3 cells via activation of the Akt-IκB-NF-κB pathway (6). Similar to ERK activation, LPA-stimulated Akt activation differs depending on the cell type. Akt responds to LPA in DU-145 and PC-3 cells, but it is constitutively active without LPA stimulation in LNCaP cells (13). The NF-κB heterodimer is also a critical transcription factor for human prostate cancer. It is constitutively activated in human prostate cancer tissue, but not in benign prostate tissues. It, therefore, indicates that constitutive active NF-κB may be due to the stimulation of LPA. Besides, LPA inhibits serum deprivation-induced autophagy in PC-3 cells (18). Together, these imply that LPA facilitates prostate cancer development by enhancing cell survivals.
LPA regulates prostate cancer cell motility
LPA regulates cell migration in various cancer cells including prostate cancer cells. In PC-3 cells, LPA induces activation of vasodilator-stimulated phosphoprotein (VASP) which subsequently mediates lamellipodia formation to initiate cell motility (19). In addition, the calcium-independent group VIA phospholipase A2β (iPLA2β) is required for LPA-induced cell migration and invasion in mouse TRAMP-C1P3 cells (22). LPA also functions in preventing the calpain-mediated proteolysis of focal adhesion kinase (FAK) in PC-3 cells (20). Calcium-dependent calpains modulate cell migration (30,31) in a process that involves degradation of FAK (32). PC-3 cells require FAK for bombesin-induced cell motility (33). Unlike LPA stimulates FAK phosphorylation in PC-3 and DU145 cells, no effect has been shown in LNCaP cells (13). Migration of LNCaP cells does not respond to LPA (23). The small GTPase Rho is important in cell movement. Activation of Rho mediates actin rearrangements, gene transcription, cell rounding, and smooth muscle contraction. It was reported that LPA stimulates Rho in PC-3 cells via LPA receptors and G12/13 proteins which directly activate PDZRhoGEFs that contain a regulator of G protein signaling (RGS) domain (21). These studies indicate that LPA promotes prostate cancer cell migration once cancer cells have become androgen-independent and highly metastatic.
Since LPA has a role in cell migration, it may have potential roles in promoting cell invasion. For instance, LPA stimulates matrigel invasion through activation of RhoA and NF-κB in PC-3 cells (7). LPA-stimulated RhoA activity leads to morphologic changes, from polygonal to round, of PC-3 cells (34). Moreover, analysis of the profile of gene expression between highly invasive and less invasive PC-3 cell sublines suggests that invasion-related molecules are involved in invasiveness of the prostate cancer (35). For instance, higher activity levels of NF-κB, activator protein 1 (AP-1) and RhoA activities as well as thrombospondin-1, interleukin-7 (IL-7), kallikrein6, MMP-1 and tissue factor were found in invasive cells and may respond to LPA. Heterodimerization of LPA1 and adhesion-linked G protein-coupled receptor (GPCR) CD97 amplify LPA-initiated Rho-dependent signaling and invasion in PC-3 cells (36). Accordingly, the RhoA signaling cascade is necessary to promote LPA-induced cell invasion in prostate cancer cells (36).
Conversely, PC-3 cells which respond to LPA in three-dimensional culture exhibit signs of epithelial-to-mesenchymal transition (EMT) in contrast to metastable acinar differentiation. LPA promotes acinar morphogenesis and blocks the disintegration of epithelial structures with the basal lamina and formation of invadopodia (37). This mechanism is through LPA1/Gα12/13/RhoA/ROCK pathway which suppresses invasive properties. Therefore, the functions of LPA in prostate cancer invasion in either 2-D or 3-D culture system need to be clarified in the future.
LPA mediates tumoral angiogenesis and lymphangiogenesis
VEGFs are important growth factors for angiogenesis and lymphangiogenesis in prostate cancer progression and metastasis. In PC-3 cells, LPA induces VEGF-A expression through the PI3K/Akt/mTOR/p70S6K and p42/p44 MAPK pathways that are similar in OVCAR-3 ovarian cancer cells (24,38). Moreover, hypoxia-inducible factor 1α (HIF-1α) participates in LPA-induced VEGF-A expression (24). HIF-1 proteins are transcription factors which are induced by hypoxia within the tumor. The HIF-1 complex is a heterodimer composed of HIF-1α and HIF-1β, which is also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) (39). The ARNT binding partner, aryl hydrocarbon receptor (AHR), inhibits LPA-induced VEGF-A (25). AHR is a basic helix-loop-helix transcription factors that function as an environmental sensor binding with dioxin-like compound and leads to nuclear localization. Translocated AHR further heterodimerizes with ARNT and leads to changes in gene transcription. AHR inhibits prostate carcinogenesis and vanadate-induced VEGF-A production in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice (40,41). Together, the inhibitory role of AHR in LPA-induced VEGF-A is due to the sequestering of ARNT from HIF-1α (25), thereby inhibiting tumoral angiogenesis.
VEGF-C is a critical lymphangiogenic factor that secreted by normal human tissues (42) and prostate cancer cells (43,44). The high expression of VEGF-C in prostate cancer results in the formation of lymphatic vessels which have been implicated in lymph node metastasis (45). However, the serum VEGF-C level cannot be a marker for prostate cancer growth because no significant differences between prostate cancer and BPH patients have been found (46). Overexpressing VEGF-C in poorly metastatic LAPC-9 cells induces tumoral lymphangiogenesis and leads to the development of metastatic lesions (47). Reduction of the key mitogenic factor androgen in prostate cancer cells upregulates VEGF-C (48) through ROS production and small GTPase RalA activation (49). In our lab, we found that LPA stimulates VEGF-C mRNA expression through binding to LPA1 and LPA3, producing ROS, and elevating lens epithelium-derived growth factor (LEDGF) in PC-3 cells (9). Further study demonstrated the PLC/PKC/NADPH oxidase (Nox) pathway controls LPA-induced ROS generation (10). VEGF-C seems to be more critical in tumoral lymphatic metastasis, but not cancer growth and LPA is involved in the expression of VEGF-C. Blockade of LPA receptors may be a potential treatment for inhibition of tumoral lymphangiogenesis as well as lymphatic metastasis.
LPA-related enzymes in prostate cancer
Due to the production of LPA in ovarian cancer (50), questions arose whether prostate cancer cells also generate and secrete LPA. Prostate cancer cells do produce and utilize LPA for themselves (12). A neuroendocrine peptide bombesin has been shown to stimulate LPA production. Electrospray ionization mass spectrometry showed that 18:1 LPA (Oleoyl-LPA) is the most abundant LPA in the prostate cancer medium. Secreted LPA from prostate cancer cells induces calcium mobilization (12). These results demonstrate that LPA is generated by prostate cancer cells and suggest that 18:1 LPA act as an autocrine mediator. LPA activates phospholipase D (PLD) in prostate cancer cells, including PC-3, DU-145, and LNCaP cells (5,13), to catalyze phospholipids (PLs) to phosphatidic acid (PA) and further may produce more LPA to stimulate the cellular response. Protein kinase C (PKC) is also involved in the LPA-induced activation of PLD in PC-3 cells (5). In addition, two important LPA-producing enzymes, LysoPLD (Autotaxin, ATX) and acylglycerol kinase (AGK), were found in prostate cancer samples (51) and seminal plasma (52). ATX and AGK are both highly expressed at the protein level in prostate cancer cells, including LNCaP, PC-3, and DU-145 cells, compared with non-neoplastic prostate cells, including PrECs and PrSCs (53). ATX is the major LPA-synthesizing enzyme for extracellular LPA production. Activated platelets are responsible for the increased levels of ATX in serum (54-56). However, the activity of ATX in prostate cancer patients has been shown no differences from that in the controls (57). Besides, there is no correlation between serum ATX activity and serum PSA concentrations. Nevertheless, ATX is not a useful diagnostic marker for prostate cancer patients. Another enzyme, AGK, is an intracellular lipid kinase that localizes to the mitochondria in epithelial cells and fibroblasts and phosphorylates monoacylglycerol and diacylglycerol to form LPA and PA, respectively (51,58). Prostate cancer patients and PC-3 cells highly express AGK. This increase results in the formation and secretion of LPA, which cross-talk with EGFR resulting in the subsequent activation of ERK1/2. AGK expression enhanced both prostate cancer cell proliferation and migration. Furthermore, the LPA degradation enzyme, prostatic acid phosphatase (PAP), was found in the seminal plasma (52). PAP is a non-specific phosphomonoesterase that is synthesized and secreted into the seminal plasma under androgenic control (59). LNCaP cells expressing endogenous PAP show a slow growth rate compared with PC-3 and DU-145 cells that lack PAP expression (60). Intriguingly, the introduction of PAP into prostate cancer cells results in decreased cell growth. Moreover, the cellular form of PAP is involved in regulating the androgen-stimulated growth of prostate cells (61). C-33 LNCaP cells that express PAP and AR are responsive to androgen stimulation, whereas C-81 LNCaP and PC-3 cells that express the functional AR but lack PAP expression are androgen-insensitive. Reintroducing cellular PAP expression can restore androgen responsiveness of these cell lines. This suggests that PAP has a role in inhibiting the proliferation of prostate cells by negatively regulating the LPA level. Clinical evidence indicates that AGK was abundantly expressed in the stroma and epithelium while PAP was predominantly localized in the epithelial cells of benign prostatic hyperplasia (BPH) (53,62). Conversely, ATX is predominantly expressed in the stroma. However, the expression of ATX does not significantly differ between normal tissue, benign gland, and cancer foci. In conclusion, LPA-degrading enzyme PAP, rather than LPA-producing enzyme ATX or AGK, may be the key player to mediate the levels of LPA in prostate cancer, and therefore affect prostate cancer cell behaviors.
General function of the expression of LPA receptors
LPA receptors are GPCRs with seven transmembrane domains that are activated by binding with LPA and subsequently initiate downstream cellular signaling cascades for different biological functions (63). Six LPA receptors have been identified and classified into two types: LPA1-3 are close relative and initially known as the endothelial differentiation gene (EDG) while LPA4-6 belong to the P2Y purinergic receptor family. LPA1 was the first to be identified (64) and the best studied LPA receptor. LPA1 activation regulates cellular events, such as cell-cell contact alteration, cell proliferation and survival, cell migration and cytoskeletal changes, calcium mobilization, and adenylyl cyclase inhibition (65-67). Lpar1/LPAR1 is widely expressed in the organs of adult mice and humans and is enriched in parts of the brain during embryonic development (67,68). Lpar1−/− mice were generated and demonstrated clear neurodevelopmental defects. A 50% of perinatal lethality of these mice may be due to olfactory deficits leading to a defect in suckling behavior (69,70). Therefore, LPA1 is involved in the nervous system development and function. In cancer cells, LPA1 mediates cell motility and metastasis. LPAR2 is highly expressed in the testis and leukocytes while Lpar2 is highly expressed in the kidney, uterus, and testis. LPAR3 is expressed in the heart, testis, prostate, pancreas, lung, ovary, and brain of human (71,72) and LPA3 is expressed in the frontal cortex, hippocampus, and amygdala (71). These suggest LPA3 may have significant functions in the brain. However, Lpar3−/− mice are viable and grossly normal in the nervous system. Lpar3−/− mice showed delays in embryo implantation as well as reduced litter size and embryo implantation suggesting a role of LPA signaling in reproduction system (73). LPA4 negatively regulates cell motility which is notably different from EDG LPA1-3 receptors that promote cell migration (74). Lpar4 is present in mouse heart, skin, thymus, bone marrow, and embryonic brain (75). Some Lpar4−/− mice show hemorrhage and prenatal lethality during embryonic development (76), but others can grow up normally (75). The abnormal formation of blood during mouse embryogenesis cause prenatal death (76) of mice. However, neither LPA5 nor LPA6 knockout mice have been generated yet. Hypotrichosis patients show LPAR6 mutation (77-79), which suggests that LPA6 is critical for the formation of human hair. The expression of LPA receptors depends on the cell type that will mediate signaling transduction in the cells.
Expression profile of LPA receptors and signaling pathway in prostate cancer
LPA receptors coupled with Gα proteins, including Gs, Gi/o, Gq/11, and G12/13, initiate a variety of signaling cascades. LPA1 interacts with three types of Gα proteins, Gi/o, Gq/11, and G12/13, which leads to the activation of downstream effectors such as mitogen-activated protein kinase (MAPK), phospholipase C (PLC), Akt, and Rho, respectively (80). Like LPA1, LPA2 couples with Gi/o, Gq/11, and G12/13 and subsequently initiates downstream effectors such as Ras, MAPK, PI3K, Rac, PLC, diacylglycerol, and Rho (67). LPA3 couples with Gi/o and Gq/11 and mediates PLC, adenyl cyclase and MAPK activation (81). LPA5 coupled with G12/13 and Gq, thereby initiate receptor internalization and elevates intracellular calcium levels (82). LPA6 is involved in cAMP accumulation and Rho-dependent cell morphology alterations through G13 (83). Therefore, the expressions of LPA receptors are important to mediate activation of the downstream signaling pathways as well as cellular events.
Prostate cancer cells highly express LPA receptors, LPA1, LPA3, and LPA6 but not LPA2, LPA4, and LPA5 (Figure 1) (13,17,84). The levels of LPA receptors are different between the androgen-dependent and androgen-insensitive prostate cancer cells (17). The LPA1 gene is dominantly expressed in PC-3 and DU-145, but not LNCaP cells. Stable expressing of LPA1 in LNCaP cells shows a response to LPA-induced cell proliferation in vitro and in vivo. LPA1 may play a role in transducing proliferative signals in prostate cancer by transducing Gα-independent signals to promote AR nuclear localization and cell proliferation (17). LPA2 is expressed in these three types of cells as well as being highly expressed in LNCaP cells (62). LNCaP cells show high levels of LPA3 in comparison to PC-3 cells while DU-145 cells do not express LPA3. Clinical evidence also indicates the importance of LPA receptors in prostate cancer (62). Both high-grade intraepithelial neoplasia (HGPIN) and cancer epithelia displayed significantly decreased levels of LPA1 mRNA compared with the benign glands. Cancer epithelia showed greater expression of LPA3 mRNA compared with the benign glands. The expression of LPA2 is high in epithelium compared to the stroma of prostate when microdissected from benign glands, BPH, high-grade PIN (PIN) and prostate cancer foci in the prostate harboring prostate cancer. However, the expression of LPA2 is not significantly modified when comparing normal tissue, benign gland, and cancer foci. Most papers have demonstrated the functions of LPA1, LPA2 and LPA3 in prostate cancer; however, the pathophysiological roles of LPA6 in prostate cancer remain unclear and needs to be clarified.
Future prospects
Tumor progression is affected by alteration in the surrounding microenvironment. A study has therefore demonstrated the intercellular cross-talk of prostate cancer cells with prostate stromal cells in response to LPA. The co-culture of human prostate stromal PS30 and epithelial LNCaP cells results in the activation of ERK in LNCaP cells and further enhanced the biophysiological activities to LPA stimulation (85). Implantation of a mixture of both cell types into nude mice reveals the physiologic relevance of the interaction between these two cells. Tumors from mice with both kinds of cells are larger compared with only mice implanted with LNCaP cells. The larger tumor is because LPA stimulates synthesis of interleukin 6 (IL-6) in PS30 cells. IL-6 controls the LPA-induced mitogenic ERK and STAT3 signaling and growth of the LNCaP cells. These results suggest that other surrounding cells such as endothelial cells or epithelial cells may also participate in cross-talk with prostate cancer cells and regulate the physiological functions.
The expression and function of LPA receptors are critical for regulating prostate cancer progression and metastasis. Therefore, a selective antagonist for LPA receptors may represent a potential therapy against tumor development. For instance, Ki16425 is a selective antagonist for LPA1 and LPA3 (86). Administering the R-stereoisomer of Ki16425, Debio 0719, into BALB/c mice with orthotopic mouse 4T1 breast cancer inhibits bone and lung metastasis from the primary tumor (53), but not tumor growth and angiogenesis. In prostate cancer, Ki16425 treatment into nude mice after subcutaneously implanting PC-3 cells inhibits heparin-binding EGF-like growth factor (HB-EGF) secretion by human PC-3 xenograft (84). HB-EGF was therefore identified as a biomarker for LPA1 activation in human prostate cancer in vitro and in vivo (84). However, Ki16425 treatment does not reduce the size of primary tumors in prostate cancer, which is the same result found in the treatment of breast cancer. The expression levels of LPA receptors do not change in tumors with Ki16425 treatment compared with the vehicle-treated group. These suggest that in vivo Ki16425 treatment does not inhibit PC-3 tumor growth or apoptosis. The functions of the blockade of LPA1/3 by Ki16425 should be further analyzed.
Compared with the EDG LPA receptors, little is known about the biological roles of the novel subtype of LPA receptors LPA4-6 in cancer. LPA4 and LPA5 are difficult to detect in prostate cancer, which suggests that expressions of both are repressed in malignant cells. Conversely, LPA6 is highly expressed (84) in prostate cancer, although the role of LPA6 has not been identified. Hence, further study is required on the biological functions of LPA6 in prostate cancer progression.
Conclusions
In this review, we summarize that LPA increases cell proliferation and promotes cell survival in advanced prostate cancer. Cell migration and invasion are also stimulated by LPA, suggesting that LPA mediates prostate cancer metastasis. Moreover, LPA stimulates VEGF-A and VEGF-C expression which may promote tumor angiogenesis and lymphangiogenesis and therefore metastasis. Moreover, these LPA-regulated cell behaviors in prostate cancer are mainly mediated through activating LPA receptors. Interestingly, advanced prostate cancer cells secret LPA and the level of secreted LPA is affected by LPA-degrading enzyme PAP rather than by LPA-producing enzyme ATX. Therefore, clarify the roles of LPA, LPA receptors, and its related enzymes in prostate cancer will help us to identify the pathological functions and molecular mechanisms in prostate cancer progression. Accordingly, targeting the specific effectors of LPA signaling in prostate cancer may contribute to the development of clinical therapeutic strategies for advanced prostate cancer in the future.
Acknowledgments
Funding: This work was supported by National Health Research Institutes, Taiwan with grant number NHRI-EX101-10130BI, National Taiwan University, Taiwan with grant number NTU 102R76263A, and Ministry of Science and Technology, Taiwan with grant number NSC 103-2311-B-002-015-.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Lysophospholipids on Immunity and Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2015.10.05). The series “Lysophospholipids on Immunity and Cancer” was commissioned by the editorial office without any funding or sponsorship. HL served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 1996;28:251-65. [PubMed]
- Huggins C. Endocrine-induced regression of cancers. Cancer Res 1967;27:1925-30. [PubMed]
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;168:9-12. [PubMed]
- Tan MH, Li J, Xu HE, et al. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin 2015;36:3-23. [PubMed]
- Qi C, Park JH, Gibbs TC, et al. Lysophosphatidic acid stimulates phospholipase D activity and cell proliferation in PC-3 human prostate cancer cells. J Cell Physiol 1998;174:261-72. [PubMed]
- Raj GV, Sekula JA, Guo R, et al. Lysophosphatidic acid promotes survival of androgen-insensitive prostate cancer PC3 cells via activation of NF-kappaB. Prostate 2004;61:105-13. [PubMed]
- Hwang YS, Hodge JC, Sivapurapu N, et al. Lysophosphatidic acid stimulates PC-3 prostate cancer cell Matrigel invasion through activation of RhoA and NF-kappaB activity. Mol Carcinog 2006;45:518-29. [PubMed]
- Hao F, Tan M, Xu X, et al. Lysophosphatidic acid induces prostate cancer PC3 cell migration via activation of LPA(1), p42 and p38alpha. Biochim Biophys Acta 2007;1771:883-92.
- Lin CE, Chen SU, Lin CC, et al. Lysophosphatidic acid enhances vascular endothelial growth factor-C expression in human prostate cancer PC-3 cells. PLoS One 2012;7:e41096 [PubMed]
- Lin CC, Lin CE, Lin YC, et al. Lysophosphatidic acid induces reactive oxygen species generation by activating protein kinase C in PC-3 human prostate cancer cells. Biochem Biophys Res Commun 2013;440:564-9. [PubMed]
- Guo C, Luttrell LM, Price DT. Mitogenic signaling in androgen sensitive and insensitive prostate cancer cell lines. J Urol 2000;163:1027-32. [PubMed]
- Xie Y, Gibbs TC, Mukhin YV, et al. Role for 18:1 lysophosphatidic acid as an autocrine mediator in prostate cancer cells. J Biol Chem 2002;277:32516-26. [PubMed]
- Gibbs TC, Rubio MV, Zhang Z, et al. Signal transduction responses to lysophosphatidic acid and sphingosine 1-phosphate in human prostate cancer cells. Prostate 2009;69:1493-506. [PubMed]
- Kue PF, Taub JS, Harrington LB, et al. Lysophosphatidic acid-regulated mitogenic ERK signaling in androgen-insensitive prostate cancer PC-3 cells. Int J Cancer 2002;102:572-9. [PubMed]
- Picascia A, Stanzione R, Chieffi P, et al. Proline-rich tyrosine kinase 2 regulates proliferation and differentiation of prostate cells. Mol Cell Endocrinol 2002;186:81-7. [PubMed]
- Liu Z, Hopkins MM, Zhang Z, et al. Omega-3 fatty acids and other FFA4 agonists inhibit growth factor signaling in human prostate cancer cells. J Pharmacol Exp Ther 2015;352:380-94. [PubMed]
- Guo R, Kasbohm EA, Arora P, et al. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology 2006;147:4883-92. [PubMed]
- Chang CL, Liao JJ, Huang WP, et al. Lysophosphatidic acid inhibits serum deprivation-induced autophagy in human prostate cancer PC-3 cells. Autophagy 2007;3:268-70. [PubMed]
- Hasegawa Y, Murph M, Yu S, et al. Lysophosphatidic acid (LPA)-induced vasodilator-stimulated phosphoprotein mediates lamellipodia formation to initiate motility in PC-3 prostate cancer cells. Mol Oncol 2008;2:54-69. [PubMed]
- Park JJ, Rubio MV, Zhang Z, et al. Effects of lysophosphatidic acid on calpain-mediated proteolysis of focal adhesion kinase in human prostate cancer cells. Prostate 2012;72:1595-610. [PubMed]
- Wang Q, Liu M, Kozasa T, et al. Thrombin and lysophosphatidic acid receptors utilize distinct rhoGEFs in prostate cancer cells. J Biol Chem 2004;279:28831-4. [PubMed]
- Li H, Zhang H, Wei G, et al. Tumor cell group via phospholipase A2 is involved in prostate cancer development. Prostate 2011;71:373-84. [PubMed]
- Hama K, Aoki J, Fukaya M, et al. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem 2004;279:17634-9. [PubMed]
- Lee J, Park SY, Lee EK, et al. Activation of hypoxia-inducible factor-1alpha is necessary for lysophosphatidic acid-induced vascular endothelial growth factor expression. Clin Cancer Res 2006;12:6351-8. [PubMed]
- Wu PY, Lin YC, Lan SY, et al. Aromatic hydrocarbon receptor inhibits lysophosphatidic acid-induced vascular endothelial growth factor-A expression in PC-3 prostate cancer cells. Biochem Biophys Res Commun 2013;437:440-5. [PubMed]
- Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 1999;402:884-8. [PubMed]
- Tokiwa G, Dikic I, Lev S, et al. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science 1996;273:792-4. [PubMed]
- Dikic I, Tokiwa G, Lev S, et al. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 1996;383:547-50. [PubMed]
- Shin SH, Kwon YW, Heo SC, et al. Krüppel-like factor 4 mediates lysophosphatidic acid-stimulated migration and proliferation of PC3M prostate cancer cells. Exp Mol Med 2014;46:e104 [PubMed]
- Dourdin N, Bhatt AK, Dutt P, et al. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem 2001;276:48382-8. [PubMed]
- Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci 2005;118:3829-38. [PubMed]
- Carragher NO, Westhoff MA, Riley D, et al. v-Src-induced modulation of the calpain-calpastatin proteolytic system regulates transformation. Mol Cell Biol 2002;22:257-69. [PubMed]
- Lacoste J, Aprikian AG, Chevalier S. Focal adhesion kinase is required for bombesin-induced prostate cancer cell motility. Mol Cell Endocrinol 2005;235:51-61. [PubMed]
- Chen Y, Wang Y, Yu H, et al. The cross talk between protein kinase A- and RhoA-mediated signaling in cancer cells. Exp Biol Med (Maywood) 2005;230:731-41. [PubMed]
- Hwang YS, Lindholm PF. Constitutive and Inducible Expression of Invasion-related Factors in PC-3 Prostate Cancer Cells. J Cancer Prev 2015;20:121-8. [PubMed]
- Ward Y, Lake R, Yin JJ, et al. LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res 2011;71:7301-11. [PubMed]
- Härmä V, Knuuttila M, Virtanen J, et al. Lysophosphatidic acid and sphingosine-1-phosphate promote morphogenesis and block invasion of prostate cancer cells in three-dimensional organotypic models. Oncogene 2012;31:2075-89. [PubMed]
- Hu YL, Tee MK, Goetzl EJ, et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst 2001;93:762-8. [PubMed]
- Rodríguez-Jiménez FJ, Moreno-Manzano V. Modulation of hypoxia-inducible factors (HIF) from an integrative pharmacological perspective. Cell Mol Life Sci 2012;69:519-34. [PubMed]
- Fritz WA, Lin TM, Cardiff RD, et al. The aryl hydrocarbon receptor inhibits prostate carcinogenesis in TRAMP mice. Carcinogenesis 2007;28:497-505. [PubMed]
- Fritz WA, Lin TM, Peterson RE. The aryl hydrocarbon receptor (AhR) inhibits vanadate-induced vascular endothelial growth factor (VEGF) production in TRAMP prostates. Carcinogenesis 2008;29:1077-82. [PubMed]
- Joory KD, Levick JR, Mortimer PS, et al. Vascular endothelial growth factor-C (VEGF-C) expression in normal human tissues. Lymphat Res Biol 2006;4:73-82. [PubMed]
- Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996;15:1751. [PubMed]
- Zeng Y, Opeskin K, Goad J, et al. Tumor-induced activation of lymphatic endothelial cells via vascular endothelial growth factor receptor-2 is critical for prostate cancer lymphatic metastasis. Cancer Res 2006;66:9566-75. [PubMed]
- Tsurusaki T, Kanda S, Sakai H, et al. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer 1999;80:309-13. [PubMed]
- Voss M, Trojan L, Steidler A, et al. Serum vascular endothelial growth factor C level in patients with prostate cancer and benign prostatic hyperplasia. Anal Quant Cytol Histol 2008;30:199-202. [PubMed]
- Brakenhielm E, Burton JB, Johnson M, et al. Modulating metastasis by a lymphangiogenic switch in prostate cancer. Int J Cancer 2007;121:2153-61. [PubMed]
- Li J, Wang E, Rinaldo F, et al. Upregulation of VEGF-C by androgen depletion: the involvement of IGF-IR-FOXO pathway. Oncogene 2005;24:5510-20. [PubMed]
- Rinaldo F, Li J, Wang E, et al. RalA regulates vascular endothelial growth factor-C (VEGF-C) synthesis in prostate cancer cells during androgen ablation. Oncogene 2007;26:1731-8. [PubMed]
- Mills GB, May C, Hill M, et al. Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. J Clin Invest 1990;86:851-5. [PubMed]
- Bektas M, Payne SG, Liu H, et al. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J Cell Biol 2005;169:801-11. [PubMed]
- Tanaka M, Kishi Y, Takanezawa Y, et al. Prostatic acid phosphatase degrades lysophosphatidic acid in seminal plasma. FEBS Lett 2004;571:197-204. [PubMed]
- Nouh MA, Wu XX, Okazoe H, et al. Expression of autotaxin and acylglycerol kinase in prostate cancer: association with cancer development and progression. Cancer Sci 2009;100:1631-8. [PubMed]
- Aoki J, Taira A, Takanezawa Y, et al. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 2002;277:48737-44. [PubMed]
- Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol 2004;15:477-89. [PubMed]
- Sano T, Baker D, Virag T, et al. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem 2002;277:21197-206. [PubMed]
- Nakamura K, Takeuchi T, Ohkawa R, et al. Serum lysophospholipase D/autotaxin may be a new nutritional assessment marker: study on prostate cancer patients. Ann Clin Biochem 2007;44:549-56. [PubMed]
- Kalari S, Zhao Y, Spannhake EW, et al. Role of acylglycerol kinase in LPA-induced IL-8 secretion and transactivation of epidermal growth factor-receptor in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2009;296:L328-36. [PubMed]
- Ostrowski WS, Kuciel R. Human prostatic acid phosphatase: selected properties and practical applications. Clin Chim Acta 1994;226:121-9. [PubMed]
- Lin MF, Garcia-Arenas R, Xia XZ, et al. The cellular level of prostatic acid phosphatase and the growth of human prostate carcinoma cells. Differentiation 1994;57:143-9. [PubMed]
- Lin MF, Meng TC, Rao PS, et al. Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. J Biol Chem 1998;273:5939-47. [PubMed]
- Zeng Y, Kakehi Y, Nouh MA, et al. Gene expression profiles of lysophosphatidic acid-related molecules in the prostate: relevance to prostate cancer and benign hyperplasia. Prostate 2009;69:283-92. [PubMed]
- Kihara Y, Maceyka M, Spiegel S, et al. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br J Pharmacol 2014;171:3575-94. [PubMed]
- Hecht JH, Weiner JA, Post SR, et al. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol 1996;135:1071-83. [PubMed]
- Ishii I, Fukushima N, Ye X, et al. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321-54. [PubMed]
- Fukushima N, Ishii I, Contos JJ, et al. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol 2001;41:507-34. [PubMed]
- Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol 2000;58:1188-96. [PubMed]
- An S, Bleu T, Hallmark OG, et al. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem 1998;273:7906-10. [PubMed]
- Contos JJ, Fukushima N, Weiner JA, et al. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci U S A 2000;97:13384-9. [PubMed]
- Weiner JA, Fukushima N, Contos JJ, et al. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci 2001;21:7069-78. [PubMed]
- Bandoh K, Aoki J, Hosono H, et al. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem 1999;274:27776-85. [PubMed]
- Im DS, Heise CE, Harding MA, et al. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol Pharmacol 2000;57:753-9. [PubMed]
- Ye X, Hama K, Contos JJ, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005;435:104-8. [PubMed]
- Lee Z, Cheng CT, Zhang H, et al. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell 2008;19:5435-45. [PubMed]
- Lee CW, Rivera R, Dubin AE, et al. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem 2007;282:4310-7. [PubMed]
- Sumida H, Noguchi K, Kihara Y, et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood 2010;116:5060-70. [PubMed]
- Pasternack SM, von Kügelgen I, Al Aboud K, et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet 2008;40:329-34. [PubMed]
- Shimomura Y, Garzon MC, Kristal L, et al. Autosomal recessive woolly hair with hypotrichosis caused by a novel homozygous mutation in the P2RY5 gene. Exp Dermatol 2009;18:218-21. [PubMed]
- Nahum S, Morice-Picard F, Taieb A, et al. A novel mutation in LPAR6 causes autosomal recessive hypotrichosis of the scalp. Clin Exp Dermatol 2011;36:188-94. [PubMed]
- Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 2003;3:582-91. [PubMed]
- Ishii I, Contos JJ, Fukushima N, et al. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol Pharmacol 2000;58:895-902. [PubMed]
- Lee CW, Rivera R, Gardell S, et al. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem 2006;281:23589-97. [PubMed]
- Yanagida K, Masago K, Nakanishi H, et al. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem 2009;284:17731-41. [PubMed]
- David M, Sahay D, Mege F, et al. Identification of heparin-binding EGF-like growth factor (HB-EGF) as a biomarker for lysophosphatidic acid receptor type 1 (LPA1) activation in human breast and prostate cancers. PLoS One 2014;9:e97771 [PubMed]
- Sivashanmugam P, Tang L, Daaka Y. Interleukin 6 mediates the lysophosphatidic acid-regulated cross-talk between stromal and epithelial prostate cancer cells. J Biol Chem 2004;279:21154-9. [PubMed]
- Ohta H, Sato K, Murata N, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol 2003;64:994-1005. [PubMed]


