Clinical outcomes of patients treated with accelerated partial breast irradiation with high-dose rate brachytherapy: Scripps Clinic experience
Introduction
Patients with early stage breast cancer have options of either mastectomy or breast conserving therapy (BCT), which consists of partial mastectomy (or lumpectomy) followed by radiation therapy to the breast. Both options have similar outcomes (local control and survival) as shown in multiple prospective randomized studies.
For the past few decades, whole-breast irradiation (WBI) has been shown to reduce the risk of breast tumor recurrence after partial mastectomy for early breast cancer. Typical WBI is given at standard dose fractionation of 1.8 to 2 Gy per day, 5 days per week, for about 5 to 5.5 weeks for a dose about 50 Gy, then a boost dose of about 10–15 Gy for another 1–2 weeks. Multiple randomized clinical trials have demonstrated the effectiveness and safety of WBI. BCT is the preferred treatment modality for many patients with early stage breast carcinoma. The major advantages of BCT are superior cosmetic outcome and the reduced emotional and psychological impact from this procedure compared with mastectomy. The principal disadvantage of breast conservation as it traditionally has been performed is the prolonged treatment duration requiring approximately 5 to 7 weeks of external beam radiation therapy which may pose substantial problems for some patients such as the elderly with limited transportation, those who live far from a radiotherapy facility, and busy working patients. Most patients receive WBI have mild side effects; however, a small number of patients developed WBI significant side effects such as skin scarring, breast fibrosis, rib fractures, lymphedema, radiation pneumonitis, cardiac toxicity, and secondary cancer from radiation. Fear of these severe toxicities and inconvenience of prolonged course of daily radiation have steered patients toward mastectomy or skipping post-op radiation after breast conserving surgery.
Over the past couple of decades, there has been growing interest in the use of accelerated partial-breast irradiation (APBI) as an alternative to WBI. APBI is defined as radiation treatment of the area around the lumpectomy cavity only (“partial breast”) over a short period of time such as 1–2 weeks with 5 to 10 treatments (“accelerated”) using higher dose per fraction than standard 1.8 to 2 Gy (“hypofractionation”). Typical dose for APBI is 34 Gy in 10 fractions or 32 Gy in 8 fractions, which is biologically equivalent to about 50 Gy in 25 fractions. APBI provides two major advantages over conventional WBI. First, it is more convenient since radiation therapy can be shortened to one to two weeks versus typical 5–7 weeks. Second, normal tissues such as the remainder of the breast, underlying muscle, ribs, lung, and heart generally will receive less radiation dose with APBI than with WBI, potentially avoiding short term side effects and long-term toxicities. However, there are several disadvantages to APBI. First, occult foci of cancer that exist far from the lumpectomy cavity and elsewhere in the breast will not be treated with APBI. Second, most of the techniques used for APBI utilize external devices (catheters or balloon) that are implanted in the breast for a short period of time. These devices and their implantation require additional cost and surgical procedures, and they can cause pain, infection, and bleeding. Given the interest in APBI, several multi-center, randomized clinical trials have been initiated to compare the effectiveness and safety of APBI and WBI.
Randomized controlled trials have previously shown that the rate of local recurrence, with appropriately selected patients, undergoing APBI is equivalent to WBI (1,2). With the growing interest of APBI in place of WBI for breast conserving treatment, a number of societies, including the American Society of Radiation Oncology (ASTRO), have published guidelines for appropriate patient selection for APBI. At the time of that publication, the ASTRO society reported more than 32,000 women in the USA had been treated with the high dose rate (HDR) brachytherapy technology, which is the most common APBI technique (3). At the ASTRO meeting this year even more prospective studies were presented and reaffirmed prior studies’ conclusions that APBI treatment had excellent toxicity profiles and cosmetic outcomes as well as reconfirming low local recurrence rates (4). We report the retrospective data gathered from a single institution over a decade for the group of early stage breast cancer patients treated at Scripps Clinic.
Materials and methods
This was a single institution retrospective chart review of female patients who were treated at the Scripps Clinic. This retrospective chart review study was approved by Scripps IRB. A total of one hundred and fifty female early stage breast cancer patients were treated with accelerated partial breast irradiation (APBI) using HDR brachytherapy between the years 2002–2013. Three patients were ultimately excluded from the calculation of breast cancer recurrence rate due to lack of follow up. Patients who were deemed possible candidates for APBI at Scripps Clinic had localized breast cancer stage 0 to IIB. Information in regards to stage, histopathology, and recurrence rate were all obtained from the Scripps Breast Cancer Registry. This registry tracks patients yearly after their diagnosis. Any recorded recurrence is confirmed by the patient’s physician or another cancer registry. Of note, the registry was unable to obtain data for approximately half of the patients after the initial one year follow up.
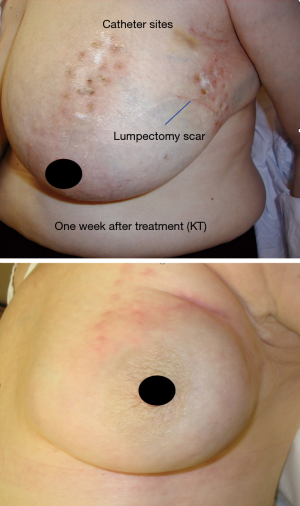
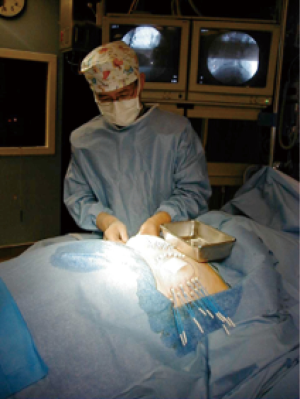
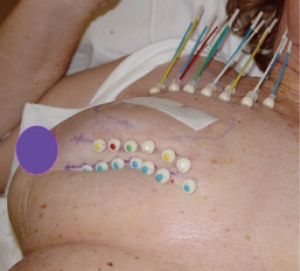
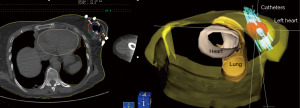
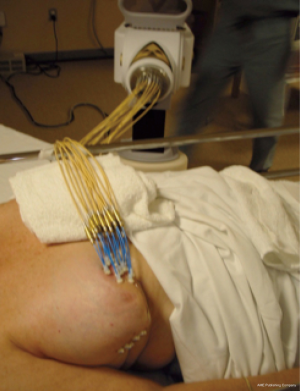
In the early period from 2002 to 2008, the patients were treated with multi-catheter interstitial HDR brachytherapy (about 80% of the patient). After 2008, the techniques were changed to balloon based such as Mammosite (Hologic, Inc., Marlborough, MA, USA) or SAVI® (strut adjusted volume implant) applicator (Cianna Medical, Aliso Viejo, CA, USA). For the patients who underwent multi-catheter interstitial HDR brachytherapy, multiple catheters were implanted into the patient’s breast right after the breast conserving surgery and clearance of surgical margin on the frozen pathology as shown in Figure 1. About 12–18 closed end lumen catheters were implanted around the lumpectomy cavity within 2 to 3 planes as shown in Figure 2. The patient underwent CT treatment planning, and the dose of 34 Gy in 10 fractions was prescribed to the lumpectomy cavity plus 1 to 1.5 cm sparing the skin and chest wall. The dose to skin and chest wall was kept under 34 Gy, and the volume of 150% hot spot was kept under 20% (Figure 3). Patient received 2 treatments per day with 6 hour interval. The patient treatment was delivered with HDR Iridium-192 Nucletron System (Figure 4) under direct supervision of the radiation oncologist and physicist. At the end of the last treatment, the catheters were removed. Figure 5 showed the patient skin at 1 and 10 weeks after the treatment.
Results (Table 1)
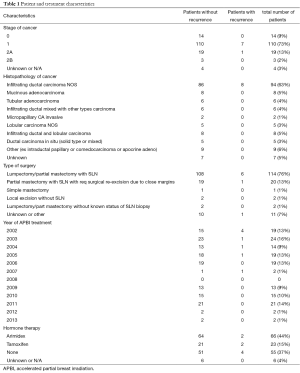
Full table
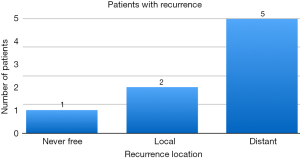
The majority of patients, 73.3%, treated at our institution had stage I disease. Fifty-nine percent of patients received hormonal treatment therapy. Of note, our institution did include a limited number of patients with lobular breast cancer or infiltrating lobular and ductal disease, 8.6% of patients, with no known recurrence amongst this group of patients. The recurrence rate is shown on Figure 6.
Discussion
At Scripps Clinic, the rate of local breast cancer recurrence after APBI with HDR brachytherapy with minimal follow up of one year was 1.4%. The recurrence rate for breast cancer at any site was found to be 5.4%. These rates are similar to rates published in other retrospective studies that have followed patients over a similar period of time (5). One limitation of the study is the lack of follow up for many patients beyond one year of treatment.
At Scripps Clinic, the modalities used for APBI HDR brachytherapy were multi-catheter interstitial brachytherapy or balloon catheter brachytherapy. Another limitation to our study is that the specific treatment modalities used for some patients were not available for all patients. However, due to the low rate of recurrence of local breast cancer it likely would not have been possible to draw any significant conclusions from rates of local recurrence even if the specific brachytherapy modality was known.
Our institution also included a small percentage of patients that were considered “cautionary” based on current guidelines. While they made up only 8.6% of the patient population, it is notable that this group did not have any local recurrences.
There are a number of ongoing studies, including the SHARE trial, that are now looking at outcomes of invasive breast cancer patients of all pathological subtypes which should help to determine if patients with less favorable pathologic subtypes, such as lobular breast cancer, may be deemed appropriate candidates for ABPI in future.
Conclusions
The experience of this single institution on more than 150 breast cancer patients during stage 0 to II treated with ABPI using HDR brachytherapy over a decade reproduces similar outcome compared to other major studies. The results from a small sample of patients who are considered “cautionary” by ASTRO yield similar outcomes as the rest of the group.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2016.01.11). HG serves as an unpaid Co-Editor-in-Chief of Translational Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective chart review study was approved by Scripps IRB and individual consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arthur DW, Winter K, Kuske RR, et al. A Phase II trial of brachytherapy alone after lumpectomy for select breast cancer: tumor control and survival outcomes of RTOG 95-17. Int J Radiat Oncol Biol Phys 2008;72:467-73. [PubMed]
- Polgár C, Fodor J, Major T, et al. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol 2013;108:197-202. [PubMed]
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. [PubMed]
- Budrukkar A, Gurram L, Upreti RR, et al. Clinical outcomes of prospectively treated 140 women with early stage breast cancer using accelerated partial breast irradiation with 3 dimensional computerized tomography based brachytherapy. Radiother Oncol 2015;115:349-54. [PubMed]
- Edwards JM, Herzberg SM, Shook JW, et al. Breast conservation therapy utilizing partial breast brachytherapy for early-stage cancer of the breast: a retrospective review from the Saint Luke's Cancer Institute. Am J Clin Oncol 2015;38:174-8. [PubMed]