
Effects of p-cresol, a uremic toxin, on cancer cells
Highlight box
Key findings
• Uremic toxin p-cresol promoted HepG2 human liver cancer cell migration and invasion.
What is known and what is new?
• Few studies have investigated the effects of p-cresol on cancer cells.
• Uremic toxin p-cresol appeared to enhance the malignant biological behaviour of HepG2 human liver cancer cells.
What is the implication, and what should change now?
• Despite accounting for only 2% of total p-cresol in the blood, p-cresol accumulates in various organs of uremic patients. Because the liver is the primary site for p-cresol metabolism, the tumour-promoting effect of p-cresol on HepG2 cells merits further investigation.
Introduction
Uremic retention solutes that accumulate in the body of a uremic patient are regarded as toxins when they exert toxic effects on various systems. Many uremic toxins have been linked to complications such as cardiovascular disease (1), immune dysfunction (2), anemia (3), malnutrition (4), bone disorder (5), and neurological disorders (6). Also, the relationship between uremia and cancer has been studied over the last 50 years. Penn et al. proposed that uremia is carcinogenic as early as the 1970s (7). Patients with impaired renal function, particularly those with uremia, appear to be at a higher risk of cancer (8-11). However, the precise mechanisms have not been thoroughly clarified.
Endogenous p-cresol, a major byproduct of tyrosine fermentation, belongs to the group of protein-bound uremic toxins that are insufficiently eliminated by dialysis therapy. It is primarily produced in the colon and metabolized in the liver. The urine is the primary route of excretion. Because of the progressive decline in renal function, p-cresol may accumulate in the circulation and organs. P-cresol concentrations in the blood, urine, liver, and kidney of uremic patients underwent hemodialysis (HD) are approximately 5- to 25-fold higher than those of the control group (12). Previous research has demonstrated that p-cresol causes apoptosis (13), necrosis (14), and senescence (15) in a variety of cells. However, few studies have been conducted to investigate the effects of p-cresol on cancer cells. A recent study found that p-cresol exhibited dose- and time-dependent toxicity to bladder cancer cells. It also promoted the invasion and migration of living cells (16). This raises the question of whether p-cresol acts similarly on other cancer cells.
Cancer is currently regarded as one of the leading causes of death in HD patients (17). Several studies have been conducted to investigate the relationship between uremia and cancer. Despite different opinions, most studies found an increased cancer risk in uremic patients (18-22). Kidney cancer (19,20) and liver cancer (21,22) are the most common cancers found in patients receiving dialysis. P-cresol can accumulate in HD patients’ organs, with 9% and 54% of free p-cresol in the kidney and liver, respectively (12). In this study, we investigated the effects of p-cresol on kidney and liver cancer cells. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2042/rc).
Methods
Cell line and cell culture
This in vitro study used 786-O human renal cancer cells and HepG2 human liver cancer cells obtained from the Chinese Academy of Sciences. 786-O cells were grown in RPMI 1640 medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS), 50 U/mL penicillin, and 100 µg/mL streptomycin. HepG2 cells were grown in DMEM/F12 complete medium (Gibco, Grand Island, New York), which was a 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 medium with a 10% FBS supplement. Every 2 to 3 days, we changed the medium. We cultured the cells in a 37 ℃ incubator with 5% CO2 humidified air until they reached the log phase. According to the previous references (16,23), different concentrations (0, 10, 20, 40, 70 µM) of p-cresol (C85751. Sigma-Aldrich) for 48 hours were chosen to treat these cells.
Cell Counting Kit-8 (CCK-8) assay for cell viability
Cell viability was quantified using the CCK-8. Cells were seeded at a density of 1×104 cells/mL in a 96-well plate. Each well received 100 µL of medium containing 10% FBS. For 48 hours, cells were treated with p-cresol at different concentrations (0, 10, 20, 40, 70 µM). Then, 10 µL of CCK-8 solution (Beyotime, Shanghai, China) was added to each well of the plate and incubated for 0.5 to 2 hours in a humidified incubator (37 ℃, 5% CO2). The absorbance was measured at 450 nm and was repeated 3 times for each well.
TUNEL assay for cell apoptosis
The apoptotic cells were identified using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Cells were dewaxed, rehydrated, and incubated for 15 to 30 minutes at 20 to 37 ℃ with a 20 µg/mL proteinase K working solution. The slides were washed with phosphate buffer saline (PBS), dried, and then incubated for one hour in the dark at 37 ℃ in a 50 µL TUNEL reaction mixture (Roche Biosciences). After washing with PBS, cells in the samples were stained with DAPI (4'6-diamidino-2-phenylindol) for DNA staining, and fluorescence microscopy (Olympus, Tokyo, Japan) was used to examine them.
Transwell assay for cell migration and invasion
To assess the migratory and invasive responses of 786-O cells and HepG2 cells to p-cresol, the two-chamber Transwell (Corning Co., Corning, NY, USA) was used. In the invasion assay, Matrigel (BD Biosciences) was inoculated evenly into the upper chamber to form a gel at 37 ℃ after being diluted in a serum-free medium. After 48 hours of p-cresol treatment, 200 µL of cell suspension (1×106 cells/mL) without serum was added to the upper compartment, followed by 600 µL of culture medium containing 10% FBS in the lower compartment. After 24 hours of incubation at 37 ℃ in humidified air containing 5% CO2, the migrated or invaded cells into the lower surface of the membrane were fixed, stained, and counted under the microscope (Olympus, Tokyo, Japan).
Statistical analysis
For data analysis, SPSS 17.0 statistical software was used. The Student’s t-test was used to compare the means between two groups. A P value <0.05 was considered statistically significant.
Results
Effects of p-cresol on the viability of HepG2 cells and 786-O cells
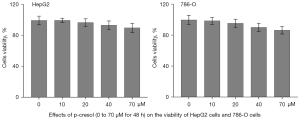
The cytotoxic effects of p-cresol on HepG2 and 786-O cells were investigated using the CCK-8 assay. For 48 hours, HepG2 and 786-O cells were treated with p-cresol at different concentrations of 0, 10, 20, 40, and 70 µM. The results showed that p-cresol at 0 to 70 µM for 48 hours had no toxic effects on HepG2 or 786-O cells (Figure 1). For the following experiment, we chose 40 µM p-cresol for 48 hours (16).
Effects of p-cresol on the apoptosis of HepG2 cells and 786-O cells
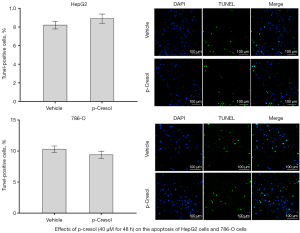
TUNEL assay and DAPI staining were used to determine apoptotic cells in HepG2 and 786-O cells after 48 hours of treatment with 40 µM p-cresol. The results showed that 40 µM p-cresol for 48 hours did not affect the apoptosis of HepG2 (P=0.5185) or 786-O cells (P=0.1012) (Figure 2).
Effects of p-cresol on the migration and invasion of HepG2 cells and 786-O cells
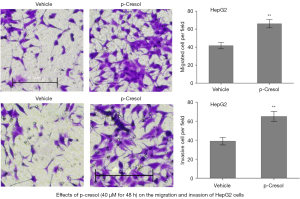
The transwell migration/invasion assay was then used to determine whether p-cresol influenced the motility of HepG2 and 786-O cells. The results showed that after 48 hours of treatment with p-cresol at a concentration of 40 µM, HepG2 cells demonstrated increased migration (P=0.0019) and invasion (P=0.0025) (Figure 3). However, p-cresol treatment did not affect 786-O cell migration (P=0.2720) (Figure 4).

Discussion
In the past 40 years, cancer has been proposed as a challenge for uremic patients (24). Cancer is currently one of the top three causes of death among uremic patients who have received HD therapy (17). Moreover, cancer is the leading cause of death in uremic patients who have cancer (9,17,18). Uremic patients have unique cancer risk factors, such as impaired immune function, chronic inflammation-malnutrition status, and persistent metabolic disorders (24-26). Also, uremia has been identified as a potent carcinogen, and its accumulation may predispose patients to cancer (7). Uremia, or ‘‘urine in the blood’’, is a medical condition that causes toxic substances to accumulate in body fluid compartments due to a progressive decrease in renal function (27). Uremic toxins are traditionally classified as small water-soluble compounds, middle molecular weight molecules, and protein-bound solutes (28,29). Cresols have received a lot of attention among the protein-bound uremic toxins (30).
P-cresol is an organic aromatic compound that belongs to the cresol class. Endogenous p-cresol is produced in the colon by bacterial fermentation of dietary tyrosine (31). Following absorption, it is metabolized and conjugated in the liver before being excreted by the kidney (32). Most of the p-cresol in the human body is sulfated into p-cresyl sulfate, and the remained is metabolized to p-cresyl glucuronide (30). Nonetheless, the concentrations of the mother compound p-cresol in HD patients’ blood, urine, and organs are several times higher than in non-HD patients (12). So, the response of cells in direct contact with p-cresol is still worth investigating, particularly in HD patients (30).
Previous research has found that high doses of p-cresol have toxic effects on various cell types in the kidney (14), heart (33), gut (31), bone (34), and immune (35), suggesting that its accumulation in uremic patients may play a role in the development of several complications. However, its effects on cancer cells are rarely investigated. Hsu et al. recently clarified the effects of p-cresol on cancer cells. They found that p-cresol had a dose- and time-dependent toxic effect on human bladder cancer TSGH8301 cells. According to their findings, p-cresol inhibited the viability of these bladder cancer cells but promoted the migration and invasion of the living ones (16).
This study aims to see if p-cresol had similar effects on other cancer cells. For this study, we used 786-O human renal cancer cells and HepG2 human liver cancer cells because kidney cancer (19,20) and liver cancer (21,22) were the most commonly reported cancers in uremic patients. Our findings showed that exposing 786-O cells and HepG2 cells to 0 to 70 µM p-cresol for 48 hours did not affect their viability or proliferation. Then, for 48 hours, we used 40 µM p-cresol to see how it affected the migration and invasion of these cancer cells. We found only the malignant biological behavior of HepG2 cells to be significantly promoted by p-cresol. Although p-cresol did not affect 786-O cells in our study, its main metabolite, p-cresyl sulphate, in other research was found to induce 786-O cell proliferation and migration in a dose (20–500 µM) and time (6–48 hours) dependent manner (23). The structural changes caused by the replacement of hydroxyl with sulphate reduce the lipophilicity of p-cresol and turn its sulphate conjugate into a hydrophilic compound, which may be the main cause of the differences in biological effects between p-cresol and p-cresyl sulphate (36). P-cresyl sulphate, for example, had a pro-inflammatory effect on unstimulated leucocytes, while p-cresol inhibited the activity of stimulated leucocytes (37). Also, while both p-cresol and p-cresyl sulphate increased the migration of bladder cancer cells, they did so via distinct signaling pathways (16,38).
Despite accounting for only 2% of total p-cresol in the blood, p-cresol accumulates in various organs of HD patients (12). So, the effects of p-cresol are attributed not only to the products of its metabolism but also to p-cresol itself (39). Because the liver is the primary site for its metabolism (40), free p-cresol is the major component of total p-cresol, followed by its conjugates (12). So, the tumor-promoting effect of p-cresol on HepG2 cells discovered in this study merits further investigation. Previous studies have shown that the risk of liver cancer in uremic patients is almost 1.5 times higher than in the general population, owing to a higher incidence of hepatitis virus infection and comorbid metabolic diseases such as diabetes mellitus (21,22,41). However, besides these risk factors shared by the general population, it is unknown whether uremia plays a role in the formation and progression of liver cancer. Our findings showed that the uremic toxin p-cresol appeared to enhance the malignant biological behavior of HepG2 human liver cancer cells. Unfortunately, we did not investigate the molecular mechanisms by which p-cresol induced HepG2 cell invasion and migration. Also, the biological function of p-cresol in animals requires further investigation.
Conclusions
Summarily, this study demonstrated that p-cresol at concentrations ranging from 0 to 70 µM for 48 hours had no significant toxicity on 786-O human renal cancer cells or HepG2 human liver cancer cells. A 48-hour treatment with 40 µM p-cresol did not affect the motility of 786-O cells. Interestingly, it promoted HepG2 cell migration and invasion.
Acknowledgments
The authors acknowledge the contributions of the other investigators in this study.
Funding: This study was funded by Shanghai Municipal Key Clinical Specialty (No. shslczdzk02501).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2042/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2042/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2042/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2042/coif). All authors report that this study was funded by Shanghai Municipal Key Clinical Specialty (No. shslczdzk02501). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lekawanvijit S. Cardiotoxicity of Uremic Toxins: A Driver of Cardiorenal Syndrome. Toxins (Basel) 2018;10:352. [Crossref] [PubMed]
- Cohen G. Immune Dysfunction in Uremia 2020. Toxins (Basel) 2020;12:439. [Crossref] [PubMed]
- Macdougall IC. Role of uremic toxins in exacerbating anemia in renal failure. Kidney Int Suppl 2001;78:S67-72. [Crossref] [PubMed]
- Sahathevan S, Khor BH, Ng HM, et al. Understanding Development of Malnutrition in Hemodialysis Patients: A Narrative Review. Nutrients 2020;12:3147. [Crossref] [PubMed]
- Fujii H, Goto S, Fukagawa M. Role of Uremic Toxins for Kidney, Cardiovascular, and Bone Dysfunction. Toxins (Basel) 2018;10:202. [Crossref] [PubMed]
- Assem M, Lando M, Grissi M, et al. The Impact of Uremic Toxins on Cerebrovascular and Cognitive Disorders. Toxins (Basel) 2018;10:303. [Crossref] [PubMed]
- Penn I, Halgrimson CG, Starzl TE. De novo malignant tumors in organ transplant recipients. Transplant Proc 1971;3:773-8. [PubMed]
- Wong G, Hayen A, Chapman JR, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol 2009;20:1341-50. [Crossref] [PubMed]
- Wong G, Staplin N, Emberson J, et al. Chronic kidney disease and the risk of cancer: an individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer 2016;16:488. [Crossref] [PubMed]
- Lowrance WT, Ordoñez J, Udaltsova N, et al. CKD and the risk of incident cancer. J Am Soc Nephrol 2014;25:2327-34. [Crossref] [PubMed]
- Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol 2010;23:253-62. [PubMed]
- Ikematsu N, Kashiwagi M, Hara K, et al. Organ distribution of endogenous p-cresol in hemodialysis patients. J Med Invest 2019;66:81-5. [Crossref] [PubMed]
- Tanaka S, Yano S, Sheikh AM, et al. Effects of uremic toxin p-cresol on proliferation, apoptosis, differentiation, and glucose uptake in 3T3-L1 cells. Artif Organs 2014;38:566-71. [Crossref] [PubMed]
- Brocca A, Virzì GM, de Cal M, et al. Cytotoxic effects of p-cresol in renal epithelial tubular cells. Blood Purif 2013;36:219-25. [Crossref] [PubMed]
- Lee JH, Yun CW, Hur J, et al. Fucoidan Rescues p-Cresol-Induced Cellular Senescence in Mesenchymal Stem Cells via FAK-Akt-TWIST Axis. Mar Drugs 2018;16:121. [Crossref] [PubMed]
- Hsu YH, Huang HP, Chang HR. The uremic toxin p-cresol promotes the invasion and migration on carcinoma cells via Ras and mTOR signaling. Toxicol In Vitro 2019;58:126-31. [Crossref] [PubMed]
- Yoo KD, Lee JP, Lee SM, et al. Cancer in Korean patients with end-stage renal disease: A 7-year follow-up. PLoS One 2017;12:e0178649. [Crossref] [PubMed]
- Chen X, Li Y, Ding X, et al. Incidence, Risk, and Prognosis of Cancer in Patients on Chronic Hemodialysis. Blood Purif 2020;49:310-21. [Crossref] [PubMed]
- Stewart JH, Buccianti G, Agodoa L, et al. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 2003;14:197-207. [Crossref] [PubMed]
- Tsuzuki T, Iwata H, Murase Y, et al. Renal tumors in end-stage renal disease: A comprehensive review. Int J Urol 2018;25:780-6. [Crossref] [PubMed]
- Chien CC, Han MM, Chiu YH, et al. Epidemiology of cancer in end-stage renal disease dialysis patients: a national cohort study in Taiwan. J Cancer 2017;8:9-18. [Crossref] [PubMed]
- Lin HF, Li YH, Wang CH, et al. Increased risk of cancer in chronic dialysis patients: a population-based cohort study in Taiwan. Nephrol Dial Transplant 2012;27:1585-90. [Crossref] [PubMed]
- Wu TK, Wei CW, Pan YR, et al. The uremic toxin p-cresyl sulfate induces proliferation and migration of clear cell renal cell carcinoma via microRNA-21/ HIF-1α axis signals. Sci Rep 2019;9:3207. [Crossref] [PubMed]
- Kjellstrand CM. Are malignancies increased in uremia? Nephron 1979;23:159-61. [Crossref] [PubMed]
- Vamvakas S, Bahner U, Heidland A. Cancer in end-stage renal disease: potential factors involved -editorial-. Am J Nephrol 1998;18:89-95. [Crossref] [PubMed]
- Mandayam S, Shahinian VB. Are chronic dialysis patients at increased risk for cancer? J Nephrol 2008;21:166-74. [PubMed]
- Glassock RJ. Uremic toxins: what are they? An integrated overview of pathobiology and classification. J Ren Nutr 2008;18:2-6. [Crossref] [PubMed]
- Vanholder R, Glorieux G, De Smet R, et al. New insights in uremic toxins. Kidney Int Suppl 2003;S6-10. [Crossref] [PubMed]
- Lisowska-Myjak B. Uremic toxins and their effects on multiple organ systems. Nephron Clin Pract 2014;128:303-11. [Crossref] [PubMed]
- Vanholder R, Bammens B, de Loor H, et al. Warning: the unfortunate end of p-cresol as a uraemic toxin. Nephrol Dial Transplant 2011;26:1464-7. [Crossref] [PubMed]
- Andriamihaja M, Lan A, Beaumont M, et al. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic Biol Med 2015;85:219-27. [Crossref] [PubMed]
- Gryp T, De Paepe K, Vanholder R, et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int 2020;97:1230-42. [Crossref] [PubMed]
- Peng YS, Lin YT, Wang SD, et al. P-cresol induces disruption of cardiomyocyte adherens junctions. Toxicology 2013;306:176-84. [Crossref] [PubMed]
- Kamprom W, Tawonsawatruk T, Mas-Oodi S, et al. P-cresol and Indoxyl Sulfate Impair Osteogenic Differentiation by Triggering Mesenchymal Stem Cell Senescence. Int J Med Sci 2021;18:744-55. [Crossref] [PubMed]
- Kawakami K, Makino I, Kato I, et al. p-Cresol inhibits IL-12 production by murine macrophages stimulated with bacterial immunostimulant. Immunopharmacol Immunotoxicol 2009;31:304-9. [Crossref] [PubMed]
- Zhu JZ, Zhang J, Yang K, et al. P-cresol, but not p-cresylsulphate, disrupts endothelial progenitor cell function in vitro. Nephrol Dial Transplant 2012;27:4323-30. [Crossref] [PubMed]
- Schepers E, Meert N, Glorieux G, et al. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 2007;22:592-6. [Crossref] [PubMed]
- Peng YS, Syu JP, Wang SD, et al. BSA-bounded p-cresyl sulfate potentiates the malignancy of bladder carcinoma by triggering cell migration and EMT through the ROS/Src/FAK signaling pathway. Cell Biol Toxicol 2020;36:287-300. [Crossref] [PubMed]
- Zhu S, Rong Y, Kiang TKL. Effects of p-Cresol on Oxidative Stress, Glutathione Depletion, and Necrosis in HepaRG Cells: Comparisons to Other Uremic Toxins and the Role of p-Cresol Glucuronide Formation. Pharmaceutics 2021;13:857. [Crossref] [PubMed]
- Gryp T, Vanholder R, Vaneechoutte M, et al. p-Cresyl Sulfate. Toxins (Basel) 2017;9:52. [Crossref] [PubMed]
- Shebl FM, Warren JL, Eggers PW, et al. Cancer risk among elderly persons with end-stage renal disease: a population-based case-control study. BMC Nephrol 2012;13:65. [Crossref] [PubMed]


