
Long-term survival of patients with intracranial metastases from thyroid cancer presenting with seizures: a case report and literature review
Introduction
Thyroid cancer has relatively low incidence and mortality. According to published statistics, its accounts for 3% of all cancers and has a mortality rate of 0.4% (1). Thyroid cancer rarely metastasizes to the brain, and there is no previous work describing a patient with thyroid cancer accompanied by seizures. However, we here report a case of intracranial metastasis from thyroid cancer presenting with generalized seizures. We further review the neurological symptoms and histopathology of intracranial metastases from thyroid cancer. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1942/rc).
Case presentation
On March 23, 2018, a 38-year-old, right-handed woman came to our hospital’s emergency department due to sudden-onset, full-body shaking with loss of consciousness lasting over 12 hours. After being admitted to the hospital, the patient experienced uncontrolled recurrent shaking and cyanosis of the lips. She was given diazepam and sodium valproate to control the seizures, mannitol to lower intracranial pressure, and other symptomatic treatment. The patient was intubated and ventilated, with ventilator-assisted spontaneous breathing to manage a drop in SpO2.
On examination, the patient had a Glasgow Coma Scale (GCS) of 1-T-5. Following stabilization of the patient’s vital signs and resolution of the seizures, a computed tomography (CT) scan of the head revealed an irregular, high-density mass in the left frontal lobe approximately 32×24 mm in size (Figure 1A). Extensive low-density changes in the bilateral frontal lobes were also observed. The patient had a past medical history of partial surgical resection of thyroid cancer 9 years prior. After thyroid surgery, the patient had been taking oral levothyroxine sodium tablets and calcium carbonate tables to inhibit thyroid stimulating hormone (TSH), and her TSH levels on admission were 0.02 mU/L. Unfortunately, postoperative pathology was unavailable, although there was suspicion that the mass might be an intracranial metastasis of thyroid cancer.
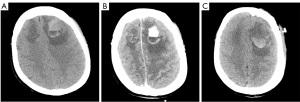
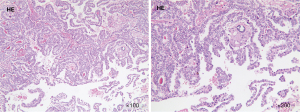
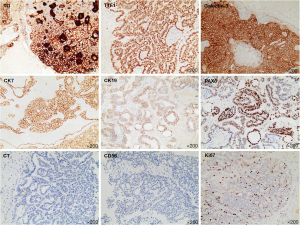
The patient was subsequently transferred to the department of neurosurgery. A magnetic resonance imaging (MRI) scan of the head was planned for surgical planning; however, the patient was unable to cooperate. Thus, we chose a shorter-duration CT-enhanced scan of the head, which showed bilateral frontal masses with ring enhancement (Figure 1B). After discussion, it was decided to perform the left frontal mass resection. Postoperative CT showed that the left frontal mass had been completely resected (Figure 1C). The patient recovered well and no abnormalities or neurological deficits were found at 2 weeks post-surgery. The histopathology results confirmed the mass as an intracranial metastasis of papillary thyroid cancer (Figure 2). Immunohistochemistry showed these tumor cells to be positive for thyroglobulin (TG), thyroid transcription factor-l (TTF1), galectin-3, cytokeratin 7 (CK7), cytokeratin 19 (CK19), and paired box 8 (PAX8) and negative for calcitonin and CD56. The Ki-67 proliferation rate was 5% (Figure 3). The whole-body CT scan revealed that the thyroid cancer had migrated to both lungs, the liver, the right subclavicular lymph nodes, and the mediastinal lymph nodes before discharge from the hospital (Figure 4). Unfortunately, the patient declined further treatment and was lost to follow-up.
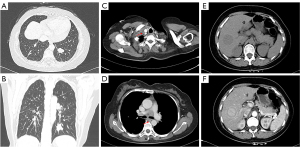
Approximately 1 year later, a large, enhanced, cystic-solid mass in the right frontal lobe and a small, enhanced mass in the right occipital lobe were observed via MRI (Figure 5A,5B). The right frontal mass was significantly larger than the previous one; however, the patient had no significant neurological symptoms. TSH was controlled with oral medication for this patient. The TSH level was 0.015 mU/L on the second admission. The frontal mass was resected completely, which was confirmed on postoperative MRI (Figure 5C,5D). The second histopathological finding also revealed intracranial metastasis of papillary thyroid cancer. The patient eventually underwent whole-brain radiotherapy beginning on September 17, 2019. The dosage schedule was 30 Gy in 10 fractions over 2 weeks. She began taking anlotinib hydrochloride on March 23, 2020, at a dose of 12 mg daily for 2 weeks with a 1-week break. Currently, the patient is still alive. The full specific treatment timeline is shown in Table 1.
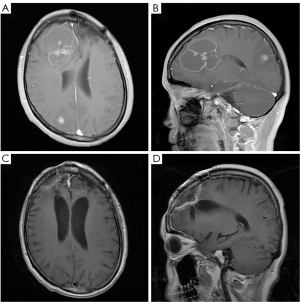
Table 1
| Date | Event |
|---|---|
| March 23, 2018 | Admitted to our hospital due to sudden full-body shaking and loss of consciousness lasting over 12 hours. Resuscitation measures included control seizures, lower intracranial pressure, tracheal intubation, and ventilator-assisted spontaneous breathing |
| March 23, 2018 | CT scan of the head revealed an irregular high-density mass in the left frontal lobe and extensive low-density change in the bilateral frontal lobes |
| March 27, 2018 | Preoperative CT-enhanced scan of the head showed bilateral frontal masses with ring enhancement |
| March 28, 2018 (first operation) | Surgical resection of the left frontal mass was performed, and the mass was resected completely |
| April 3, 2018 | Whole-body CT scan revealed that thyroid cancer had migrated to both lungs, the liver, right subclavicular lymph nodes, and mediastinal lymph nodes |
| April 10, 2018 | Histopathology results confirmed intracranial metastasis of papillary thyroid cancer. Patient discharged from hospital without neurological deficits but declined further treatment and was lost to follow-up |
| May 8, 2019 | The patient was admitted to our hospital again due to an MRI scan showing a large enhanced cystic-solid mass in the right frontal lobe, which was significantly larger than the previous one, and a small enhanced mass in the right occipital lobe |
| May 13, 2019 (second operation) | Surgical resection of the right frontal mass was performed. Postoperative CT revealed no new bleeding |
| May 25, 2019 | The patient recovered well and was discharged from hospital |
| September 17, 2019 | The patient started receiving whole-brain radiotherapy. The dosage schedule was 30 Gy in 10 fractions over 2 weeks |
| March 23, 2020 | The patient started taking anlotinib hydrochloride. It was taken at a dose of 12 mg daily continuously for 2 weeks with a 1-week break |
| June 20, 2022 | As of the last follow-up, the patient is still alive |
CT, computed tomography; MRI, magnetic resonance imaging; Gy, Gray.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
With the ninth highest incidence among all cancers, thyroid cancer is a relatively uncommon tumor type. Moreover, the mortality rate of thyroid cancer is quite low, but its incidence has gradually increased in recent years (1). Intracranial metastases from thyroid cancer (4.7%) are extremely rare and significantly less common than are lung (53.4%), bone (28.1%), and liver (8.3%) metastases (2,3). The cerebral hemispheres, cerebellum, and brainstem are the most common sites of intracranial metastases from thyroid cancer (2). A single-center study of 20 patients indicated a close link between intracranial metastasis of thyroid cancer with TERT and BRAF-V600E mutations (4).
The patient in this case study initially presented with generalized seizures and was subsequently diagnosed with intracranial metastases from primary thyroid cancer. Seizures are one of the most common symptoms of brain tumors, which are seen in approximately 30% of patients with brain tumor (5). The underlying mechanism may be related to the brain tumor’s effect on the surrounding cerebral cortex. The risk of seizures is closely related to the type and location of brain tumor (6). For cases of intracranial metastases, seizures can occur in up to 35% of patients (5). To our knowledge, only 3 retrospective studies (7-9) of intracranial metastases in thyroid cancer have discussed neurological symptoms (Table 2). In our article, neurological symptoms are classified as focal deficits, signs of increased intracranial pressure, neuropsychological symptoms, and seizures. Of the 67 patients, 25 presented with focal deficits, 14 with signs of increased intracranial pressure, 8 with neuropsychological symptoms, and 20 with no symptoms. Notably, no seizures have been reported in previous retrospective studies of intracranial metastases from thyroid cancer.
Table 2
| Authors (year of publication) | No. of patients | No. of neurological symptoms | ||||
|---|---|---|---|---|---|---|
| Focal deficits | Signs of increased intracranial pressure | Neuropsychological symptoms | Seizures | No symptoms | ||
| Choi et al. (2016) (7) | 37 | 11 | 10 | 4 | 0 | 12 |
| Saito et al. (2016) (8) | 20 | 10 | 2 | 3 | 0 | 5 |
| Slutzky-Shraga et al. (2018) (9) | 10 | 4 | 2 | 1 | 0 | 3 |
| Total | 67 | 25 | 14 | 8 | 0 | 20 |
Histopathology results confirmed intracranial metastases of papillary thyroid cancer in this patient. There are 4 histopathological subtypes of thyroid cancers: well-differentiated thyroid cancers, poorly differentiated thyroid cancers, medullary thyroid cancers, and other rare thyroid tumors. Among them, well-differentiated thyroid cancers can be divided into papillary thyroid cancers, follicular thyroid cancers, and Hurthle cell thyroid cancer. Table 3 shows the retrospective studies of thyroid cancer and intracranial metastases, including histopathology results of differentiated thyroid cancers (4,7-17). In these studies, we found that papillary thyroid cancers accounted for 56.03% of all differentiated thyroid cancers; however, the incidence of papillary thyroid cancers is approximately 80–90% of all types of thyroid cancers (18). Out of the 307 cases, 17.26% were follicular thyroid cancers, 15.64% were poorly differentiated thyroid cancers, and 2.28% were Hurthle cell thyroid cancer. These results are not completely consistent with epidemiological findings of thyroid cancer.
Table 3
| Authors (year of publication) | No. of patients | Included thyroid cancer patients | No. of histopathology | |||
|---|---|---|---|---|---|---|
| Papillary | Follicular | Hurthle cell | Poorly differentiated | |||
| Biswai et al. (1994) (10) | 5 | Differentiated | 1 | 4 | 0 | 0 |
| Chiu et al. (1997) (11) | 47 | All | 32 | 0 | 0 | 0 |
| Samuel et al. (1997) (12) | 15 | Well differentiated | 4 | 10 | 1 | 0 |
| Salvati et al. (2001) (13) | 12 | All | 3 | 3 | 0 | 0 |
| McWilliams et al. (2003) (14) | 16 | All | 10 | 2 | 1 | 1 |
| Henriques de Figueiredo et al. (2014) (15) | 21 | Differentiated | 12 | 5 | 0 | 4 |
| Choi et al. (2016) (7) | 37 | Differentiated | 32 | 3 | 0 | 2 |
| Saito et al. (2016) (8) | 25 | Differentiated | 18 | 7 | 0 | 0 |
| Slutzky-Shraga et al. (2018) (9) | 10 | Nonmedullary | 3 | 3 | 0 | 4 |
| Gomes-Lima et al. (2018) (16) | 24 | Differentiated | 8 | 8 | 1 | 6 |
| Hong et al. (2018) (17) | 16 | All | 7 | 4 | 1 | 2 |
| Osborne et al. (2019) (4) | 79 | Differentiated | 42 | 4 | 3 | 29 |
| Total (%) | 307 (100%) | 172 (56.03%) | 53 (17.26%) | 7 (2.28%) | 48 (15.64%) | |
For the patient in this case study, we used a treatment plan of bilateral frontal mass resection followed by whole brain radiotherapy and a tyrosine kinase inhibitor (TKI) regimen. Available treatment options reported in the literature include surgical resection, radiotherapy, radioactive iodine therapy, gamma knife, and TKI. Patients treated with surgical resection, radiotherapy, or both have a significantly longer median overall survival time than do untreated patients (7,9,15). Moreover, surgical resection combined with radiotherapy is superior to radiotherapy alone and is recommended as the first choice for treatment (19). The efficacy of gamma knife or radioactive iodine therapy appears to be limited (14). This is associated with very low uptake of radioactive iodine by intracranial metastatic lesions due to a reduced expression of the sodium iodide synthesizer (20-22). In a retrospective chart review by Gomes-Lima et al., the median overall survival time of intracranial metastases patients treated with TKIs increased from 4.7 to 27.2 months (16).
Another study reported a median overall survival time after thyroid cancer intracranial metastasis of 7.1 months (15), which is significantly shorter than that of pulmonary and bone metastasis (2). In addition, the survival time of patients with multi-organ metastasis has been found to be shorter than that of patients with single-organ metastasis (2,15). According to previous reports, poor prognosis is associated with the following risk factors: advanced age (23), complete resection of the primary tumor (23), histological grade (24), lymph node metastasis (24), number of intracranial metastases (7), time from diagnosis of thyroid cancer to intracranial metastasis (4), and Karnofsky Performance Status (8). Although this patient had several risk factors, at the time of writing, she is still alive. It has been over 13 years since her thyroid cancer resection and 51 months since she was diagnosed with intracranial metastases from papillary thyroid cancer. Her length of survival might be due to the effective and prompt treatment.
In summary, this was a rare case of intracranial metastases from a primary thyroid cancer with generalized seizures. A complete resection of bilateral frontal masses was performed, and the patient was further treated with whole-brain radiotherapy and TKIs. Via literature review, we analyzed the relevant neurological symptoms and related histopathology. Although intracranial metastases from thyroid cancer are rare, clinicians should pay attention to the possibility of intracranial metastases when a patient has a history of thyroid cancer and is experiencing seizures.
Acknowledgments
All authors are grateful to the patient for the consent to report this case.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1942/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1942/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Toraih EA, Hussein MH, Zerfaoui M, et al. Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease. Cancers (Basel) 2021;13:1625. [Crossref] [PubMed]
- Sampson E, Brierley JD, Le LW, et al. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 2007;110:1451-6. [Crossref] [PubMed]
- Osborne JR, Kondraciuk JD, Rice SL, et al. Thyroid Cancer Brain Metastasis: Survival and Genomic Characteristics of a Large Tertiary Care Cohort. Clin Nucl Med 2019;44:544-9. [Crossref] [PubMed]
- van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 2007;6:421-30. [Crossref] [PubMed]
- Krajewski S, Wójcik M, Harat M, et al. Influence of Epilepsy on the Quality of Life of Patients with Brain Tumors. Int J Environ Res Public Health 2021;18:6390. [Crossref] [PubMed]
- Choi J, Kim JW, Keum YS, et al. The Largest Known Survival Analysis of Patients with Brain Metastasis from Thyroid Cancer Based on Prognostic Groups. PLoS One 2016;11:e0154739. [Crossref] [PubMed]
- Saito F, Uruno T, Shibuya H, et al. Prognosis After Brain Metastasis from Differentiated Thyroid Carcinoma. World J Surg 2016;40:574-81. [Crossref] [PubMed]
- Slutzky-Shraga I, Gorshtein A, Popovitzer A, et al. Clinical characteristics and disease outcome of patients with non-medullary thyroid cancer and brain metastases. Oncol Lett 2018;15:672-6. [PubMed]
- Biswal BM, Bal CS, Sandhu MS, et al. Management of intracranial metastases of differentiated carcinoma of thyroid. J Neurooncol 1994;22:77-81. [Crossref] [PubMed]
- Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab 1997;82:3637-42. [Crossref] [PubMed]
- Samuel AM, Shah DH. Brain metastases in well-differentiated carcinoma of the thyroid. Tumori 1997;83:608-10. [Crossref] [PubMed]
- Salvati M, Frati A, Rocchi G, et al. Single brain metastasis from thyroid cancer: report of twelve cases and review of the literature. J Neurooncol 2001;51:33-40. [Crossref] [PubMed]
- McWilliams RR, Giannini C, Hay ID, et al. Management of brain metastases from thyroid carcinoma: a study of 16 pathologically confirmed cases over 25 years. Cancer 2003;98:356-62. [Crossref] [PubMed]
- Henriques de Figueiredo B, Godbert Y, Soubeyran I, et al. Brain metastases from thyroid carcinoma: a retrospective study of 21 patients. Thyroid 2014;24:270-6. [Crossref] [PubMed]
- Gomes-Lima CJ, Wu D, Rao SN, et al. Brain Metastases From Differentiated Thyroid Carcinoma: Prevalence, Current Therapies, and Outcomes. J Endocr Soc 2018;3:359-71. [Crossref] [PubMed]
- Hong YW, Lin JD, Yu MC, et al. Outcomes and prognostic factors in thyroid cancer patients with cranial metastases: A retrospective cohort study of 4,683 patients. Int J Surg 2018;55:182-7. [Crossref] [PubMed]
- Boone RT, Fan CY, Hanna EY. Well-differentiated carcinoma of the thyroid. Otolaryngol Clin North Am 2003;36:73-90. viii. [Crossref] [PubMed]
- Nahed BV, Alvarez-Breckenridge C, Brastianos PK, et al. Neurosurgery 2019;84:E152-5. [Crossref] [PubMed]
- Lee HS, Yoo H, Lee SH, et al. Clinical characteristics and follow-up of intracranial metastases from thyroid cancer. Acta Neurochir (Wien) 2015;157:2185-94. [Crossref] [PubMed]
- Spitzweg C, Bible KC, Hofbauer LC, et al. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol 2014;2:830-42. [Crossref] [PubMed]
- Haugen BR, Kane MA. Approach to the thyroid cancer patient with extracervical metastases. J Clin Endocrinol Metab 2010;95:987-93. [Crossref] [PubMed]
- Mazzaferri EL. An overview of the management of papillary and follicular thyroid carcinoma. Thyroid 1999;9:421-7. [Crossref] [PubMed]
- Mazzaferri EL. Long-term outcome of patients with differentiated thyroid carcinoma: effect of therapy. Endocr Pract 2000;6:469-76. [Crossref] [PubMed]


