
Down-regulation of FBP1 in lung adenocarcinoma cells promotes proliferation and invasion through SLUG mediated epithelial mesenchymal transformation
Highlight box
Key findings
• FBP1 inhibits the progression of lung adenocarcinoma cells by inhibiting Slug mediated EMT processes.
What is known and what is new?
• FBP1, as a gluconeogenic enzyme, has been reported to mediate metabolic reprogramming in cancer cells.
• In this study, FBP1 was also shown to inhibit the EMT process mediated by Slug to suppress lung adenocarcinoma cell invasion and proliferation.
What is the implication, and what should change now?
• As a tumor suppressor gene, FBP1 has shown some non-metabolism-related regulatory effects and can be used as a potential target for tumor treatment.
Introduction
Lung cancer is one of the most common types of malignant tumors in the world and has the highest cancer-related mortality (1). Non-small cell lung cancer (NSCLC) is the most common pathological pattern of lung cancer, which accounts for over 80% of cases. Lung adenocarcinoma is one of the most common pathologic types in NSCLC, accounting for about 40–50% of NSCLC cases (2). In recent years, there have been many studies on NSCLC that have developed many new markers with diagnostic value (3-5), as well as new therapies. Unfortunately, the overall 5-year survival rate of NSCLC is still as low as 16% (2). Also, t should not be ignored that patients with early mortality failed to receive treatment and derive prognostic markers during initial screening. Lung cancer is a highly heterogeneous disease, so it is necessary to continuously explore its underlying mechanisms and discover more biomarkers as potential therapeutic targets.
Cellular glucose metabolism is one of the processes that maintain normal biological function. Aerobic glycolysis in mitochondria is the main pathway of adenosine triphosphate (ATP) production in most normal cells. Unlike normal cells, tumor cells have been found to be more dependent on anaerobic glycolysis even under aerobic conditions, a phenomenon known as the Warburg effect (6). Anaerobic glycolysis is significantly less efficient in producing ATP than aerobic glycolysis, so tumor cells need to take up more glucose to maintain their energy requirements, and large amounts of glucose also produce various precursors, free energy and reduction equivalents for cell proliferation during metabolism. This metabolic reprogramming of tumor cells has been the focus of cancer research, many recent studies have also verified the role of metabolic reprogramming in lung cancer progression (7-9), but the specific mechanism is still not well understood.
Fructose-1,6-bisphosphatase 1 (FBP1) is the main subtype of Fructose-1,6-bisphosphatase (FBPase), whose function is to convert fructose-1, 6-diphosphate (F-1, 6-BP) into fructose-6-phosphate (F-6-P) in the process of gluconeogenesis. At present, many studies have shown that overexpression of FBP1 can reverse glycolytic metabolism and enhance oxidative phosphorylation in tumor (10,11). Conversely, loss of FBP1 may lead to the progression of several cancers (10,12-14). In addition, FBP1 has been reported to have functions other than mediating cell metabolism. In a recent study, overexpression of FBP1 reversed the EMT process in lung cancer by inhibiting the transcription factor SLUG, thereby inhibiting tumor invasion and metastasis (15). However, whether FBP1 mediates epithelial-mesenchymal transformation (EMT) regulation in lung adenocarcinoma and the specific mechanisms remains unknown. In this study, we investigated the role of FBP1 in mediating metabolic reprogramming and EMT in lung adenocarcinoma cell lines and identified FBP1 as a potentially valuable therapeutic target. We present the following article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2200/rc).
Methods
Cell culture
The human lung cancer cell lines, A549 (SCSP-503), H1299 (TCHu160), H838 (SCSP-588), SW1573 (BS-C1152378), H1975 (TCHu193) and a normal human bronchial epithelial cell line Beas-2B (SCSP-5067), were obtained from the Cell Bank of China Science Academy (Shanghai, China). A549, H1299, H838, SW1573, H1975 and Beas-2B cell lines were maintained in RPMI-1640 medium (CORNING, Mediatech, Inc., Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS, PAN-Biotech, Aidenbach, Germany) at 37 ℃ under a humidified atmosphere containing 5% CO2.
Cells transfection
The siRNAs targets to FBP1 and control vector were purchased from GenePharma (Shanghai, China). The siRNAs were transfected into lung cancer cell lines using jetPRIME transfection reagent (PolioPlus, Illkirch, France) according to the manufacturer’s instructions. After 48 h transfection, the cells were harvested for subsequent experiments. The siRNA targeting FBP1 are as follows: 5'-CCAACGACCUGGUUAUGAATT-3' (si-FBP1#1), 5'-GGGUAAAUAUGUGGUCUGUTT-3' (si-FBP1#2), 5'-GGCAUCUAUAGAAAGAAAUTT-3' (si-FBP1#3).
Commercial lentiviral particles were supplied by Hanbio Biotechnology Co., Ltd. (Shanghai, China). For stably knockdown of FBP1 expression, the shRNA target sequence for FBP1 (5'-CCGGATGTTGGAAGATCCATCAAGGCTCGAGCCTTGATGGATCTTCCAACATTTTTTG-3') was inserted into a lentivirus expression vector (pHBLV-U6-MCS-CMV-ZsGreen-PGK-PURO). The coding sequence of human FBP1 was amplified and cloned into a lentiviral expression vector, pHBLV-CMV-MCS-3FLAG-EF1-ZsGreen-T2A-PURO. The A549 and H1299 cells were transfected with HBLV-FBP1 or the control vector. The H838 cell was transfected with pHBLV- FBP1-shRNA or scramble sequence. Stably transfected cell lines were selected using puromycin after 48 h transfection.
Metabolic assays
To detect metabolites, commercial detecting kits were used under the optimal conditions according to the protocols provided by the manufacturers: Glucose uptake of lung cancer cells were measured using the Glucose Uptake Cell-Based Assay Kit (Cayman Chemical, Ann Arbor, MI, USA); Intracellular ATP of lung cancer cells were determined using the ATP content detection kit (Solarbio, Beijing, China). Cell lysates were harvested (1×105 cell each sample) and all assays were performed in 96-well plates. BioTek Synergy Neo2 plate reader (BioTek Instruments) was used to measure absorbance, and the results were normalized and repeated three times.
Methyl thiazolyl tetrazolium (MTT) cell proliferation assay
2×103 cells were seeded into each well of 96-well plates with six replicates for each group. At the 0, 24, 48, 72 and 96 h, 20 µL of MTT stock solution (5 mg/mL, Solarbio) was added into each well and incubated for 4 h at the 37 ℃ incubator. Subsequently, the supernatant was removed and 100 µL DMSO was added to dissolve the formazan crystals. The optical density at 490 nm (OD490) was measured, and the results were normalized and repeated three times.
EdU staining
5×105 cells were seeded into a 6-well plates and cultured overnight. 20 µM EdU working solution (Beyotime, Shanghai, China) was pre-configured and added to each well in 1:1 volume for a final concentration of 10 µM. Cells were incubated for 2 h at the 37 ℃. Subsequently, the culture was terminated and fixed with methanol at room temperature for 15 min. Methanol was removed and 500 µL 0.2%Triton X-100 was added to each well. Cells were incubated to perforate the cells by gently shaking the plate at room temperature for 10 min. The dyeing reaction solution was prepared according to the proportion of the manufacturer. Subsequently, the supernatant was removed and 100 µL reaction solution was added into each well. Cells were incubated to trigger the click reaction by gently shaking the plates at room temperature in the dark for 30 min. Hoechst 33342 dye (Thermos-Fisher Scientific, Waltham, MA, USA) was used for nuclei staining. Images were taken using FV3000 Fluorescence Confocal Microscopy (Olympus, Tokyo, Japan).
Transwell assay
In general, 1×105 cells were seeded into the upper wells (CORNING) pre-coated with 15% Matrigel (CORNING) and filled with FBS-free RPMI-1640 medium, during which the lower wells were filled with complete RPMI-1640 medium. Cells were incubated at 37 ℃ for 24 hours. The cells inside the upper wells were carefully wiped off and the upper wells were fixed with paraformaldehyde for 15 min, followed by staining with 0.5% crystal violet for 30 min at room temperature. Five independent experiments were performed.
Wound healing assay
Cells were cultured in 6-well plates until they covered the entire well. Subsequently, the cells were scratched with a 200 µL pipette tip to result in a wound and six fixed observation points were marked. The images were photographed under a microscope at 0 and 24 h after scratching, and the wound width was measured, respectively. Five independent experiments were performed.
Western blot
Total proteins used for Western blot were extracted from corresponding cells using the RIPA Buffer (Beyotime) in the presence of Protease (Biosharp, Anhui, China). After denaturation, the protein was separated by SDS-PAGE, and transferred onto PVDF membrane (Millipore, Boston, MA, USA). Subsequently, the PVDF membrane was blocked with 3% BSA (Biosharp) for 1 hour, and incubated with corresponding primary antibody: FBP1 (1:2,000; Proteintech, Rosemont, IL, USA, 12842-1-AP), PFKP (1:1,000; CST, Boston, MA, USA, #12746), PFKFB3 (1:1,000; CST, #13123); PKM2 (1:1,000; CST, #4053); GLUT1 (1:1,000; CST, #73015); HK2 (1:1,000; CST, #2867); N-cadherin (1:1,000; CST, #13116); E-cadherin (1:1,000; CST, #3195); Vimentin (1:1,000; CST, #5741); Slug (1:1,000; CST, #9585); Snail (1:1,000; CST, #9585); Twist (1:1,000; CST, #69366); Claudin-1 (1:1,000; CST, #13255); the cellular internal control β-actin (1:2,000; Proteintech, 81115-1-RR) at 4 ℃ overnight. Subsequently, the PVDF member was rinsed and incubated with corresponding secondary antibody labeled with HRP at 37 ℃ for 45 min. Finally, the PVDF membrane was incubated with ECL reagent (Biosharp), followed with exposure. The bands were analyzed with ImageJ software.
Statistical analysis
All statistical analyses were performed with SPSS 26.0 statistical software (SPSS, IBM) and GraphPad Prism 8.0 Software (GraphPad software, La Jolla, CA, USA). Data are presented as mean ± standard deviation (SD). P values were calculated using a two-tailed Student’s t-test between two groups, or one-way analysis of variance (ANOVA) between multiple groups unless otherwise noted. For all the analyses, a two-sided P value of <0.05 were considered statistically significant.
Results
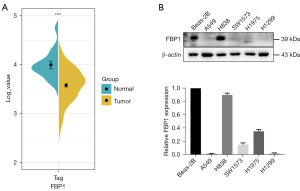
FBP1 expression is down-regulated in lung adenocarcinoma
To investigate the expression pattern of FBP1 in lung adenocarcinoma, we collected data on the expression level of FBP1 in lung adenocarcinoma samples (N=515) and normal tissues (N=49) from TCGA data (Figure 1A). We found that FBP1 was significantly down-regulated in lung adenocarcinoma (P<0.001). We further identified FBP1 expression levels in different lung adenocarcinoma cell lines (A549, H838, SW1573, H1975, and H1299). The results showed that the expression level of FBP1 in most lung adenocarcinoma cell lines except H838 was significantly lower than that in normal human bronchial epithelial cell line Beas-2B (Figure 1B). The endogenous low FBP1 expression cells A549 and H1299 were used to be transfected with pHBLV-FBP1 or control vector, and the endogenous high FBP1 expression cell H838 was used to be transfected with pHBLV- FBP1-shRNA or scramble control.
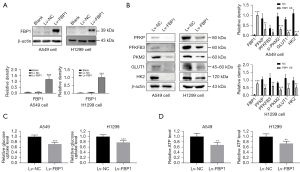
Overexpression of FBP1 inhibits glycolysis and intracellular ATP production
To investigate the role of FBP1 on lung adenocarcinoma cell, we constructed A549 and H1299 cell lines with stable overexpression of FBP1 and verified the expression of FBP1 at protein level by western blot (Figure 2A). To investigate whether overexpression of exogenous FBP1 causes metabolic reprogramming in lung adenocarcinoma cells, we conducted western blot to identify the changes in glycolysis-related protein expression levels after overexpression of FBP1 in H1299 and A549 cells. The results indicated that the expression levels of PFKP, PFKFB3, PKM2, GLUT1 and HK2 were decreased in the FBP1 overexpression group compared with the control group (Figure 2B). To further verify whether FBP1 overexpression affects the efficiency of glucose metabolism, glucose uptake rates of H1299 and A549 cells were measured. We found that overexpression of FBP1 inhibited glucose uptake (Figure 2C). In addition, we wanted to investigate whether overexpression of FBP1 interferes with energy production of lung adenocarcinoma cells. By measuring the intracellular ATP content, we found that the ATP content in the FBP1 overexpression group was significantly lower than that in the control group (Figure 2D).
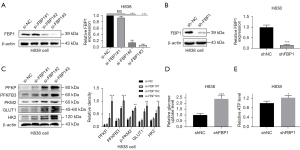
Knocking down FBP1 promotes glycolysis and intracellular ATP production
To further confirm the above findings, we used siRNA to knock down endogenous FBP1 levels in H838 cells, and constructed cell lines with stable FBP1 knockdown (Figure 3A,3B). We detected the glycolysis-related protein expression levels of endogenous FBP1 in H838 cells by western blot after instantaneous knockdown for 48 h, and found that the expressions of PFKP, PFKFB3, PKM2, GLUT1 and HK2 were all up-regulated (Figure 3C), which was contrary to the result of overexpression (Figure 2B). Similarly, glucose uptake rate and intracellular ATP content of H838 cells after stable knockdown of FBP1 were detected. Interestingly, FBP1 knockdown increased glucose uptake and intracellular ATP content (Figure 3D,3E), as opposed to overexpression (Figure 2C,2D).
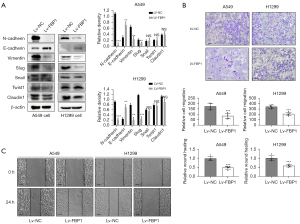
Overexpression of FBP1 reverses epithelial mesenchymal transformation
To explore the role of FBP1 in EMT, we detected the expression level of EMT-related proteins in H1299 and A549 cells after overexpression of FBP1. The results showed that compared with the control group, the expression level of epithelial related markers E-cadherin was increased in the FBP1 overexpression group, while the expression level of mesenchymal related markers N-cadherin and Vimentin was down-regulated. Among the EMT transcription factors (EMT-TFs), the expression level of Slug was down-regulated, while the expression level of Snail, Twist1 and Claudin1 did not change significantly (Figure 4A). To confirm the effect of FBP1 overexpression on cell invasiveness, Transwell assay was performed on H1299 and A549 cells. We found that the number of cells migrating the Matrigel and upper membrane was significantly reduced in the FBP1 overexpression group compared to the control group (Figure 4B). At the same time, we also conducted wound healing assay, and found that the distance of wound healing of cells in the FBP1 overexpression group was significantly lower than that in the control group 24 h after scratching (Figure 4C).
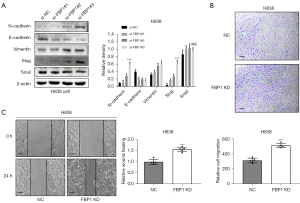
Knockdown of FBP1 promotes epithelial mesenchymal transformation
We also investigated the effect of endogenous FBP1 knockdown on EMT of lung adenocarcinoma cells. In H838 cell, FBP1 was knocked down instantaneously and EMT-related proteins were detected by WB. Epithelial related markers E-cadherin was down-regulated after FBP1 knockdown, while mesenchymal related markers N-cadherin and Vimentin was up-regulated after FBP1 knockdown. Among the EMT-TFs, Slug was up-regulated after FBP1 knockdown while Snail did not change significantly (Figure 5A). By Transwell assay, we confirmed that the invasiveness of H838 was enhanced in the group with FBP1 stably knockdown (Figure 5B). A similar trend has also been observed in the wound healing assay, that is, the stable knockdown of FBP1 can enhance the migration ability of H838 cells (Figure 5C).
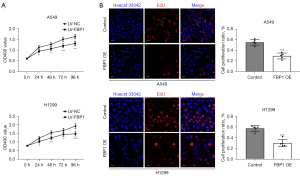
Overexpression of FBP1 inhibits proliferation of lung adenocarcinoma cells
To investigate whether FBP1 plays a role in lung adenocarcinoma cell proliferation, MTT assay and EdU staining was performed in H1299 and A549 cells. The results showed that cell proliferation was inhibited in the FBP1 overexpression group compared with the control group within 96 h after cell adherent culture (Figure 6A). By staining BOTH EdU and Hoechst 33342 for H1299 and A549 cells, we found that the percentage of EdU positive cells, that is, cells in the proliferating state, was significantly lower in the FBP1 overexpression group than in the control group (Figure 6B).
Discussion
Although a variety of new diagnosis and therapy strategy have been developed for lung cancer, its long-term survival rate is still not ideal, among which tumor recurrence and metastasis are the most important factors affecting survival. Therefore, more needs to be done to explore the molecular mechanisms of lung cancer to find more potential biomarkers.
In recent years, metabolic reprogramming as a driver of cancer development has attracted extensive attention and has been proven to be involved in the development of cancer cells (16-19). Cancer cell metabolism is characterized by enhanced glycolysis, increased glucose uptake, energy production, and the production of a large number of precursors required for cell proliferation. As a rate-limiting enzyme of gluconeogenesis, FBP1 can antagonize the rate of glycolysis in normal cells. The loss of FBP1 expression may promote cancer progression by mediating metabolic reprogramming (14,20). However, the role of FBP1 in lung adenocarcinoma is still rarely reported. By analyzing the expression levels of lung adenocarcinoma samples from the TCGA database, we found that FBP1 is down-regulated in lung adenocarcinoma. Similarly, we extracted proteins from a variety of lung adenocarcinoma cell lines and verified the loss of FBP1 expression in most lung adenocarcinoma cell lines through western blot assay. Moreover, we found that overexpression of exogenous FBP1 inhibited the expression of glycolytic enzymes, and also reduced glucose uptake and ATP production in lung adenocarcinoma cells. Knockdown of endogenous FBP1 showed the opposite effect. These results suggest that FBP1 can also antagonize glycolysis and reverse metabolic reprogramming in lung adenocarcinoma.
EMT is considered to be a key process in initiating the metastasis cascade because it enables cancer cells to acquire more aggressive characteristics (21,22). Recent studies have suggested that changes in tumor metabolic status may be involved in the regulation of EMT process. A study from Recouvreux et al. found that nutritional stress due to glutamine deprivation resulted in EMT in ductal pancreatic adenocarcinoma (PDAC) cells (23). Another study showed that FBP1 was an independent predictor of overall survival and disease-free survival in patients with gastric cancer. Overexpression of FBP1 inhibited the proliferation and invasion of gastric cancer cells. Mechanistically, FBP1 inhibits cancer by inhibiting EMT (15). Our study also revealed that overexpression of FBP1 up-regulated epithelial and down-regulated mesenchymal markers in lung adenocarcinoma. Knocking down FBP1 had the opposite effect. Interestingly, among the EMT-TFs, overexpression or knocking down FBP1 mainly affected the expression level of Slug. These results suggested that FBP1 mediated the antagonistic effect of EMT mainly by inhibiting Slug.
Of course, there are limitations to this study. For example, in this study, we used the data from TCGA database to compare the expression level of FBP1 in lung adenocarcinoma tissues and normal tissues, rather than strictly paired adjacent normal tissues, which may lead to some statistical differences. In addition, due to various factors, we failed to verify our hypothesis at the level of animal experiments, which will be supplemented in our subsequent experiments.
Conclusions
In summary, the present study demonstrated that FBP1 is low-expressed in lung adenocarcinoma and FBP1 can be used as one of the potential clinical targets through inhibiting glycolysis, cell invasion and proliferation by inhibiting Slug mediated EMT processes.
Acknowledgments
Funding: This research was supported by Zhejiang Provincial Natural Science Foundation of China (No. LY18H160021).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2200/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2200/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2200/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017;12:1109-21.
- Giannos P, Kechagias KS, Gal A. Identification of Prognostic Gene Biomarkers in Non-Small Cell Lung Cancer Progression by Integrated Bioinformatics Analysis. Biology (Basel) 2021;10:1200. [Crossref] [PubMed]
- Sayeeram D, Katte TV, Bhatia S, et al. Identification of potential biomarkers for lung adenocarcinoma. Heliyon 2020;6:e05452. [Crossref] [PubMed]
- Lu M, Fan X, Liao W, et al. Identification of significant genes as prognostic markers and potential tumor suppressors in lung adenocarcinoma via bioinformatical analysis. BMC Cancer 2021;21:616. [Crossref] [PubMed]
- Warburg O. On respiratory impairment in cancer cells. Science 1956;124:269-70. [Crossref] [PubMed]
- Huang Y, Chen Z, Lu T, et al. HIF-1α switches the functionality of TGF-β signaling via changing the partners of smads to drive glucose metabolic reprogramming in non-small cell lung cancer. J Exp Clin Cancer Res 2021;40:398. [Crossref] [PubMed]
- Lin S, Li Y, Wang D, et al. Fascin promotes lung cancer growth and metastasis by enhancing glycolysis and PFKFB3 expression. Cancer Lett 2021;518:230-42. [Crossref] [PubMed]
- Nie M, Yao K, Zhu X, et al. Evolutionary metabolic landscape from preneoplasia to invasive lung adenocarcinoma. Nat Commun 2021;12:6479. [Crossref] [PubMed]
- Dong C, Yuan T, Wu Y, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 2013;23:316-31. [Crossref] [PubMed]
- Chen R, Li J, Zhou X, et al. Fructose-1,6-Bisphosphatase 1 Reduces (18)F FDG Uptake in Hepatocellular Carcinoma. Radiology 2017;284:844-53. [Crossref] [PubMed]
- Jin X, Pan Y, Wang L, et al. Fructose-1,6-bisphosphatase Inhibits ERK Activation and Bypasses Gemcitabine Resistance in Pancreatic Cancer by Blocking IQGAP1-MAPK Interaction. Cancer Res 2017;77:4328-41. [Crossref] [PubMed]
- Hirata H, Sugimachi K, Komatsu H, et al. Decreased Expression of Fructose-1,6-bisphosphatase Associates with Glucose Metabolism and Tumor Progression in Hepatocellular Carcinoma. Cancer Res 2016;76:3265-76. [Crossref] [PubMed]
- Yang J, Wang C, Zhao F, et al. Loss of FBP1 facilitates aggressive features of hepatocellular carcinoma cells through the Warburg effect. Carcinogenesis 2017;38:134-43. [PubMed]
- Li J, Wang Y, Li QG, et al. Downregulation of FBP1 Promotes Tumor Metastasis and Indicates Poor Prognosis in Gastric Cancer via Regulating Epithelial-Mesenchymal Transition. PLoS One 2016;11:e0167857. [Crossref] [PubMed]
- Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer 2016;16:635-49. [Crossref] [PubMed]
- Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab 2016;23:27-47. [Crossref] [PubMed]
- Yeh HW, Hsu EC, Lee SS, et al. PSPC1 mediates TGF-β1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis. Nat Cell Biol 2018;20:479-91. [Crossref] [PubMed]
- Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 2015;17:351-9. [Crossref] [PubMed]
- Li B, Qiu B, Lee DS, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature 2014;513:251-5. [Crossref] [PubMed]
- Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016. Cell 2016;166:21-45. [Crossref] [PubMed]
- Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol 2015;25:675-86. [Crossref] [PubMed]
- Recouvreux MV, Moldenhauer MR, Galenkamp KMO, et al. Glutamine depletion regulates Slug to promote EMT and metastasis in pancreatic cancer. J Exp Med 2020;217:e20200388. [Crossref] [PubMed]


