
Successful treatment with osimertinib for nonmucinous bronchioloalveolar carcinoma with massive bronchorrhea and respiratory failure: a case report and literature review
Introduction
Bronchioloalveolar carcinoma (BAC; now defined as adenocarcinoma in situ; AIS), is a rare subtype of adenocarcinoma comprising 3–6% of lung cancers (1). BAC is characterized by its peripheral growth along the alveolar septa outside the pulmonary parenchyma and pleural invasion.
Bronchorrhea is defined as excess production of respiratory secretions (>100 mL/day), and has been described in bronchiectasis, tuberculosis, and chronic bronchitis. It is also present as a non-specific manifestation of BAC (2). Patients with BAC develop acute respiratory failure (ARF) caused by massive bronchorrhea, usually resulting in death. Due to the risk of missed diagnosis or misdiagnosis of BAC in its early stages and delay in treatment, clinical research on the diagnosis and management of this type of lung carcinoma has become essential.
We report a case of BAC with massive bronchorrhea and ARF that was first misdiagnosed as pneumonia. The methods used for correct diagnosis and successful treatment are discussed. We present the following case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1853/rc).
Case presentation
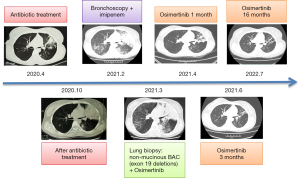
A 38-year-old woman presented on April 29, 2020 with chronic cough and sputum production, which had persisted for 10 months. She had no history of chronic pulmonary disease, diabetes mellitus, or hypertension and was a never-smoker. She was diagnosed with pneumonia at a local hospital and administered antibiotics. The symptoms were not significantly relieved by the treatment with progressive dyspnea and production of white foamy sputum occurred, especially at night, for 4 months. Bronchoalveolar lavage fluid (BALF) and sputum samples were obtained using bronchoscopy for next-generation sequencing (NGS) testing, which showed Streptococcus. During this time, the patient had recurrent fever of >40 ℃. She was treated with ceftriaxone and levofloxacin. The sputum production progressively worsened to 400 mL/day. She had no chest pain, hemoptysis, or weight loss. She was admitted to our hospital because of worsening dyspnea on February 24, 2021.
On initial physical examination, the patient showed cyanosis of the lips and respiratory distress. She had a temperature of 38.5 ℃, respiratory rate of 27 breaths/min, blood pressure of 132/83 mmHg, pulse rate of 112 beats/min, and arterial oxygen saturation of 82% in room air. Lung auscultation revealed diffused, moist rales. The cardiovascular, abdominal, and musculoskeletal examinations were unremarkable.
Laboratory analyses revealed C-reactive protein level of 12.36 mg/L and serum procalcitonin of 0.081 ng/mL. Arterial blood gas analysis showed hypoxemia (pH =7.49, PCO2 =30 mmHg, PO2 =63 mmHg) during 55% high-flow nasal cannula therapy. Routine blood count, serum urea, and electrolyte levels were normal. The level of carcinoembryonic antigen was 9.33 ng/mL. Other tumor markers such as squamous cell carcinoma antigen and neuron-specific enolase were within normal limits. Initial chest computed tomography (CT) revealed high lobar density and multiple nodules in the upper left lung (Figure 1A). Six months (October 29, 2020) after initial diagnosis, mass consolidation with air bronchograms and multiple nodules in the left lung were identified (Figure 1B). When the patient was admitted, multiple nodules, exudation, consolidation, and pulmonary cysts were seen in both lungs (Figure 1C).
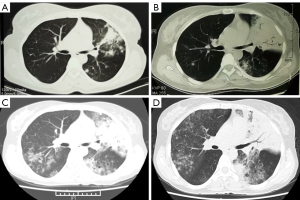
One day after admission, flexible bronchoscopy revealed many white, foamy, watery exudates in the bilateral airways. No neoplasm, hemorrhage, or necrosis was found. Also, tests for malignant cells, acid-fast bacilli, and fungal smear were negative. Pseudomonas aeruginosa and streptococcal pharyngitis were determined using BALF-NGS testing. The patient already had respiratory failure; therefore, imipenem was administered because of the initial diagnosis of severe pneumonia. The fever was relieved, but the watery sputum and dyspnea remained. Twelve days after admission (March 7, 2021), chest CT showed multiple nodules, pulmonary cysts, and consolidation with air bronchograms in both lungs, which were more severe than that at admission (Figure 1D).
Due to the unresolved issues of watery sputum and dyspnea, the patient required noninvasive mechanical ventilation (FiO2 65%) for hypoxemic respiratory failure. Bronchoscopy revealed that watery secretions filled the distal airways (Figure 2A). Cell culture, cell count, and cytology in BALF were unremarkable. Respiratory secretions were 1.5–2 L daily (Figure 2B). Despite antibiotic treatment and mechanical ventilation, oxygenation did not improve. Percutaneous CT-guided biopsy was performed 2 days later. Following tissue fixation, sectioning, and histochemical staining, histological examination showed non-mucinous BAC (Figure 2C).
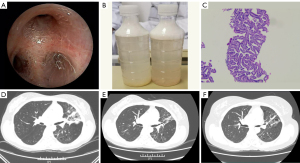
Based on the above findings, the patient was diagnosed with non-mucinous BAC. EGFR mutations (exon 19 deletions) were tested using NGS, so the patient was prescribed osimertinib (80 mg daily). The watery sputum and dyspnea were significantly relieved within 1 week. Chest CT demonstrated that the multiple nodules and the consolidation in both lungs were reduced in the 1-month (Figure 2D), 3-month (Figure 2E), and 16-month (Figure 2F) follow-up examinations, respectively. The patient was followed up, and her progression-free survival (PFS) had reached 16 months by July, 2022 (Figure 3).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Literature review
We searched the English literature with the terms of “respiratory failure”, “bronchioloalveolar carcinoma”, “bronchorrhea”, “osimertinib” via PubMed database from January 1990 to December 2021, and 11 cases with ARF were retrieved: seven (63.6%) male and four (36.4%) female (Table 1). The chest radiographic finding showed consolidation (72.7%), diffuse nodular (9%) and infiltrative shadows (9%). Two patients receiving epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) treatment had a good outcome.
Table 1
| Case/year | Gender/age (y) | Country | Manifestation | Symptoms | Survival time | Histopathology | Radiographic findings | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Hiratsuka/1998 (3) | M/54 | Japan | RF | Bronchorrhea | 11-month | NS | NS | Clarithromycin + beclomethasone | Death |
| Nonogaki/1997 (4) | M/68 | Japan | RF | Dyspnea | 6-month | Papillary adenocarcinoma | Diffuse nodular | Chemotherapy | Death |
| Nakajima/2002 (5) | M/64 | Japan | RF | Bronchorrhea | NS | Mucinous BAC | Consolidation | Corticosteroids | Death |
| Chang/2003 (6) | M/54 | China | RF | Bronchorrhea | NS | Lepidic predominant BAC | Consolidation | Gefitinib | Good |
| Kambayashi/2004 (7) | M/69 | Japan | RF | Hemoptysis | NS | Mucin-producing BAC | Infiltration shadow | Lobectomy | Good |
| Cobo Dols/2006 (8) | M/61 | Spain | RF | Dyspnea | NS | Nonmucinous BAC | Consolidation | Chemotherapy | Good |
| Venkata/2009 (9) | M/74 | America | RF | Dyspnea | 1-month | Lepidic predominant BAC | Consolidation | NS | Death |
| Yokouchi/2012 (10) | F/60 | Japan | RF | Dyspnea | 8-month | Mucinous BAC | Consolidation | Lobectomy | Death |
| Nguyen/2014 (11) | F/26 | America | RF | Dyspnea | 5-day | Lepidic predominant BAC | Consolidation | NS | Death |
| Boubaya/2014 (12) | F/84 | France | RF | Dyspnea | 3-month | Lepidic predominant BAC | Consolidation | NS | Death |
| Roeder/2017 (2) | F/60 | America | RF | Bronchorrhea | NS | Lepidic predominant BAC | Consolidation | Octreotide + erlotinib | Good |
| Present case | F/38 | China | RF | Bronchorrhea | NS | Nonmucinous BAC | Condensation | Osimertinib | Good |
BAC, bronchioloalveolar carcinoma; RF, respiratory failure; NS, not stated.
Discussion
BAC is considered a rare subtype of non-small cell lung cancer. Based on the American Thoracic Society (ATS)/European Respiratory Society (ERS)/International Association for the Study of Lung Cancer (IASLC) classification guidelines from 2011 pulmonary adenocarcinoma is now classified into lepidic predominant adenocarcinoma, atypical adenomatous hyperplasia, AIS, and minimally invasive adenocarcinoma. The term BAC has been replaced with AIS (13), which was distinguished from other subtypes of adenocarcinoma (14,15). BAC patients tend to be younger, with a greater prevalence in women (≥50%) and non-smokers (30–50%). Issues including whether there is an increased incidence of BAC in recent years and uncertainties regarding long-term survival remain unresolved (16).
The diagnosis of BAC should take into account its unique epidemiology as well as the clinical presentation, radiological findings, and histopathological examinations. Typical manifestations include cough, production of watery sputum, and progressive dyspnea. The overall pathophysiology of bronchorrhea in BAC is not yet known. The possible causes are associated with hypersecretion by bronchial gland cells, excessive synthesis of mucin leakage into the airways by EGFR overexpression, and abnormal transport of transepithelial chloride and water (17). Significant mucosecretion usually occurs due to infection and inflammatory stimuli. Respiratory failure caused by massive bronchorrhea has been reported in few cases (2,18). In the present case, the patient was misdiagnosed with pneumonia, and she was given antibiotic therapy. Bronchorrhea and hypoxemic respiratory failure progressively worsened during antibiotic therapy. Although bronchorrhea may be a symptom of BAC, it cannot be the sole basis of diagnosis. Bronchoscopy should be performed to exclude common conditions such as pulmonary edema, lung infection, or alveolar hemorrhage. BAC should be considered if characteristic bronchorrhea or respiratory failure is present.
Due to the difficulty in differentiating symptoms and most clinicians’ lack of familiarity with BAC, CT is recommended to detect any pulmonary issues. The CT images of BAC patients are complex, typically with solitary nodules, consolidation, and multiple or diffuse nodules with a ground-glass appearance (19). The size and density of the nodules can be an important indicator of the invasiveness of BAC. Consolidation with air bronchograms in chest CT images is often considered a sign of pneumonia, focal inflammation, or focal fibrosis. Non-mucinous BAC usually presents as ground-glass nodules with a solid component, whereas mucinous BAC shows more pneumonia-like consolidation or infectious cavitation (20). In the present case, multiple nodules and consolidation were visible on chest radiography. This could have been caused by mucin or other liquids filling the alveolar structures by cancer tissue infiltration, resulting in consolidation. Therefore, the radiographic manifestations vary at different stages of disease progression. A persistent pneumonic pattern or an isolated non-resolving opacity with peripheral density should be considered a potential first sign of BAC (21).
Invasive examinations, including bronchoscopy and lung biopsy, can assist in the diagnosis of BAC after an initial CT scan. In the present case, although streptococci were found using NGS testing, a lung biopsy was required because of the unrelieved symptoms and the lesions visible in the imaging while the patient was given antibiotic therapy. Complete histopathological evaluation of lung biopsy is essential to assess the invasiveness of BAC.
Currently, there are few clinical trials that systematically evaluate the most effective treatments for bronchorrhea in BAC. How to manage severe, persistent bronchorrhea is poorly understood, and currently relies on clinicians’ experience and case reports. EGFR mutations in BAC have been reported, making erlotinib or gefitinib first-choice therapies (22,23). The treatment of bronchorrhea in BAC is closely related to EGFR overexpression, which regulates mucin production by goblet cells and surfactant phospholipid synthesis in the lungs (24). This association may explain the successful treatment of BAC-associated bronchorrhea with EGFR-TKI. To our knowledge, osimertinib was used for the first time for bronchorrhea and ARF in a patient with nonmucinous BAC, and a reduction in bronchorrhea and improved respiratory symptoms were observed within 1 week. Several case reports have described the use of a macrolide and glucocorticoid (3), inhaled indomethacin (25), and octreotide (26) for treatment of bronchorrhea with BAC. However, there is no evidence regarding the impact on survival or respiratory failure for these treatments.
We performed a literature review including 11 cases with ARF (Table 1). The age of the patients ranged from 26 to 84 years. Consolidation was the most common radiographic finding. Lepidic predominant pattern lung adenocarcinomas was common, and only one patient had a previously reported non-mucinous growth pattern. The patient had respiratory failure due to dyspnea without bronchorrhea. Moreover, chemotherapy in conjunction with lobectomy was not always an effective treatment for BAC, and patients had different prognoses. Three patients died within 3 months as a result of misdiagnosis and no initial treatment. However, EGFR-TKI had a good outcome in surviving patients. More clinical data are required for further treatment analysis.
Although we were successful in treating this patient, there are a few noteworthy points. First, BAC may be misdiagnosed as pneumonia from chest imaging or atypical symptoms. Second, bronchoscopy and lung biopsy were essential in diagnosis. Third, EGFR-TKI may have a beneficial treatment outcome. One limitation was the lack of sufficient clinical research to evaluate the treatment strategy, drug efficacy and survival period of BAC.
Conclusions
In conclusion, the symptoms of BAC should be comprehensively considered, including all typical symptoms, radiological manifestations, and histological characteristics. Careful CT analysis is often neglected and can lead to misdiagnosis. Further procedures, including bronchoscopy and lung biopsy, are also necessary to properly diagnose BAC. Early diagnosis and EGFR-TKI therapy may be effective and improve disease outcomes in patients with BAC, thus clinicians should become familiar with its symptoms.
Acknowledgments
Funding: This work was supported by the Chongqing Natural Science Foundation (No. cstc2020jcyj-msxmX0008) and Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1853/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1853/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chakraborty RK, Sharma S. Bronchoalveolar Cancer. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing, 2022.
- Roeder NL, Marshall JD, Britto CJ. A Woman in Her 60s With Lung Adenocarcinoma Presents With Copious Watery Sputum and Respiratory Failure. Chest 2017;152:e143-6. [Crossref] [PubMed]
- Hiratsuka T, Mukae H, Ihiboshi H, et al. Severe bronchorrhea accompanying alveolar cell carcinoma: treatment with clarithromycin and inhaled beclomethasone. Nihon Kokyuki Gakkai Zasshi 1998;36:482-7. [PubMed]
- Nonogaki T, Morishita M, Shimizu N, et al. Diffuse bronchiolo-alveolar cell carcinoma that produced both amylase and CA19-9. Nihon Kyobu Shikkan Gakkai Zasshi 1997;35:426-31. [PubMed]
- Nakajima T, Terashima T, Nishida J, et al. Treatment of bronchorrhea by corticosteroids in a case of bronchioloalveolar carcinoma producing CA19-9. Intern Med 2002;41:225-8. [Crossref] [PubMed]
- Chang GC, Yang TY, Wang NS, et al. Successful treatment of multifocal bronchioloalveolar cell carcinoma with ZD1839 (Iressa) in two patients. J Formos Med Assoc 2003;102:407-11. [PubMed]
- Kambayashi T, Ono N, Noguchi T, et al. Bronchioloalveolar carcinoma with acute respiratory failure due to hemoptysis; report of a case. Kyobu Geka 2004;57:1161-4. [PubMed]
- Cobo Dols M, Gil Calle S, Alés Díaz I, et al. Bronchiolitis obliterans organizing pneumonia simulating progression in bronchioloalveolar carcinoma. Clin Transl Oncol 2006;8:133-5. [Crossref] [PubMed]
- Venkata C, Mireles JA, Venkateshiah SB. Refractory hypoxemic respiratory failure due to adenocarcinoma of the lung with predominant bronchioloalveolar carcinoma component. Respir Care 2009;54:1496-9. [PubMed]
- Yokouchi H, Murata K, Murakami M, et al. A case of diffuse pneumonic type of mucinous adenocarcinoma treated with reduction surgery. Gan To Kagaku Ryoho 2012;39:2396-8. [PubMed]
- Nguyen E, Hakimi M, Chavoshan B, et al. Lepidic predominant adenocarcinoma with aerogenous spread of mucin in a young patient -- a case report. Exp Mol Pathol 2014;96:400-4. [Crossref] [PubMed]
- Boubaya A, Gomez E, Guerder A, et al. Bronchioloalveolar carcinoma: an exceptional cause of diffuse lung disease in a patient with acute myeloid leukemia. Rev Mal Respir 2014;31:259-62. [Crossref] [PubMed]
- Truini A, Santos Pereira P, Cavazza A, et al. Classification of different patterns of pulmonary adenocarcinomas. Expert Rev Respir Med 2015;9:571-86. [Crossref] [PubMed]
- Pruitt AA. Epidemiology, Treatment, and Complications of Central Nervous System Metastases. Continuum (Minneap Minn) 2017;23:1580-600. [Crossref] [PubMed]
- Garfield DH, Cadranel JL, Wislez M, et al. The bronchioloalveolar carcinoma and peripheral adenocarcinoma spectrum of diseases. J Thorac Oncol 2006;1:344-59. [Crossref] [PubMed]
- Glanville AR, Wilson BE. Lung transplantation for non-small cell lung cancer and multifocal bronchioalveolar cell carcinoma. Lancet Oncol 2018;19:e351-8. [Crossref] [PubMed]
- Popat N, Raghavan N, McIvor RA. Severe bronchorrhea in a patient with bronchioloalveolar carcinoma. Chest 2012;141:513-4. [Crossref] [PubMed]
- Kurahara Y. Massive Bronchorrhea. Intern Med 2021;60:2155-6. [Crossref] [PubMed]
- Giti R, Hosseinzadeh M. Efficacy of Bronchial Washing and Brushing Cytology in the Diagnosis of Non-Neoplastic Lung Diseases. Acta Med Iran 2017;55:636-41. [PubMed]
- Carvalho AS, Cuco CM, Lavareda C, et al. Bronchoalveolar Lavage Proteomics in Patients with Suspected Lung Cancer. Sci Rep 2017;7:42190. [Crossref] [PubMed]
- Kim JE, Eom JS, Kim WY, et al. Diagnostic value of microRNAs derived from exosomes in bronchoalveolar lavage fluid of early-stage lung adenocarcinoma: A pilot study. Thorac Cancer 2018;9:911-5. [Crossref] [PubMed]
- West HL, Moon J, Wozniak AJ, et al. Paired Phase II Studies of Erlotinib/Bevacizumab for Advanced Bronchioloalveolar Carcinoma or Never Smokers With Advanced Non-Small-cell Lung Cancer: SWOG S0635 and S0636 Trials. Clin Lung Cancer 2018;19:84-92. [Crossref] [PubMed]
- Sanz Rubiales A, de la Cruz V, Berezo JÁ, et al. Erlotinib or gefitinib as first-choice therapy for bronchorrhea in bronchioloalveolar carcinoma. J Pain Symptom Manage 2014;47:e7-9. [Crossref] [PubMed]
- Takeyama K, Dabbagh K, Lee HM, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A 1999;96:3081-6. [Crossref] [PubMed]
- Homma S, Kawabata M, Kishi K, et al. Successful treatment of refractory bronchorrhea by inhaled indomethacin in two patients with bronchioloalveolar carcinoma. Chest 1999;115:1465-8. [Crossref] [PubMed]
- Pahuja M, Shepherd RW, Lyckholm LJ. The use of octreotide to manage symptoms of bronchorrhea: a case report. J Pain Symptom Manage 2014;47:814-8. [Crossref] [PubMed]


