
Contrast enhanced ultrasound guided biopsy shows higher positive sampling rate than conventional ultrasound guided biopsy for gastrointestinal stromal tumors diagnosis
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common subepithelial mesenchymal neoplasms in the gastrointestinal tract (1). GISTs can occur anywhere throughout the gastrointestinal tract from the esophagus to the anus; however, they are most common in the stomach (50% to 60%) and jejunum/ilium (25% to 30%). Duodenum (5%), colorectum (5% to 10%), and esophagus (1%) are less common sites (2). These masses are frequently found incidentally on imaging for other reasons; however, patients might also present with abdominal pain, bleeding, or symptoms of a mass effect (3,4). The differential diagnosis is quite broad, including leiomyoma, leiomyosarcoma, undifferentiated sarcomas, lipoma, carcinoid tumor, granular cell tumor, gastrointestinal schwannoma, and neurofibroma. The specific diagnosis of GIST is based on immunocytochemistry. The results of immunohistochemistry tests for GISTs have been reported to be positive for KIT (CD117; 95%), CD34 antigen (70%), smooth muscle actin (30–40%), desmin (<5%), and S-100 protein (<5%) (5).
Virtually all GISTs have the potential for malignant behavior, even those two cm or less in size with bland histological features (6). The main treatment for localized GISTs is surgical resection. For advanced unresectable tumors, GISTs respond poorly to conventional cytotoxic chemotherapy agents and radiation therapy. A target therapy agent, imatinib mesylate (Glivec; Novartis Pharma), is the recommended first-line treatment for recurrence or metastatic GISTs (7). Neoadjuvant imatinib therapy should be considered for unresectable GISTs to avoid significant morbidity or loss of organ function. Many reports have been published on this approach to convert an unresectable mass to one that is surgically approachable or to reduce the morbidity of a procedure (8).
GISTs are grossly soft and fragile tumors with a theoretic risk of tumor hemorrhage and dissemination during biopsy. According to the current consensus of management of GIST, preoperative biopsy is not generally recommended for a resectable lesion in which there is a high suspicion for GIST. However, in the presence of suspected metastatic disease or large locally advanced lesions, a biopsy is indicated to confirm the diagnosis before initiation of imatinib therapy. Image-guided percutaneous biopsies carry the theoretical risk of rupture of the tumor capsule with peritoneal spread of disease. A laparoscopic surgical biopsy also carries the risk of port site metastasis and is not recommended for the diagnosis of GISTs. Endoscopic biopsy is preferred over a percutaneous biopsy; however, conventional endoscopic biopsy using biopsy forceps may not be is effective in obtaining sufficient tissue from the submucosal tumor to confirm the diagnosis. An endoscopic snare biopsy might result in perforation and should be avoided for submucosal tumors (9,10).
However, the actual risk of peritoneal seeding, needle tract seeding, or tumor bleeding of percutaneous biopsies had never been fully evaluated. Yeh et al. reported no needle tract seeding or procedure-related tumor bleeding was seen after percutaneous biopsies (11). Furthermore, the failure rate of endoscopic biopsy was higher than that of the percutaneous biopsy group (11). Endoscopic biopsy is more suitable for GISTs with direct mucosal invasion or for those closely contiguous with gastrointestinal mucosa (12). Compared with a conventional endoscopic biopsy, ultrasound (US) guided percutaneous biopsy is a simple and straightforward procedure requiring only local anesthesia. Imaging-guided percutaneous biopsy is particularly indicated for exophytic tumors, GISTs in the jejunum and ileum, and metastatic tumors anywhere in the chest wall, abdomen, and pelvis (11).
In any case, biopsy can obtain non-representative samples and lead to false-negative diagnosis. This is caused by tissue inhomogeneity. For GIST, guidance by conventional US might not be able to identify non-liquefied necrotic tissue in large tumors, leading to unsuccessful biopsies (13). Contrast enhanced ultrasound (CEUS) with micro-bubble based contrast agents (SonoVue®, Bracco, Italy) allows visualization of the macro- and micro- vascularization of various parenchyma and tumors (14-17). CEUS has enabled delimitation of the necrotic areas from the vascularized regions of the tumors. The guarantee of tissue viability is more likely when targeting is performed using this technique. Previous studies have shown the value of CEUS guided biopsy in liver tumors. However, till now no study has investigated the value of CEUS guidance for GIST biopsies. The purpose of this study was to compare the diagnostic accuracy of conventional ultrasound (US) guided vs. contrast-enhanced ultrasound (CEUS)-guided core needle biopsy for GISTs. In our series, all patients were scheduled for Imatinib treatment as palliative therapy or neoadjuvant therapy before surgery.
Methods
Patients
The study subjects included consecutive 53 patients with a clinical diagnosis of GIST and scheduled for Imatinib treatment and who underwent core needle biopsies guided by US or CEUS between September 2011 and July 2015. The patients included 29 men and 24 women, aged 27–78 years old (mean: 56 years). Twenty patients had incidental discovery of GIST without symptoms during a health checkup, and the others had symptoms such as dyspepsia (n=8), abdominal distention and pain (n=17), or obstruction of the intestinal or urinary tract (n=8). Nine patients (17.0%) had metastatic recurrence after surgical resection of the primary tumors. All the patients had no contra-indications to percutaneous biopsy include bleeding tendency and no safe puncture path. Informed consent was obtained from all patients. The study was approved by the ethics committee of Cancer Hospital & Institute, Peking Union Medical College & Chinese Academy of Medical Sciences. The ID of the approval is NCC2013S-006. All patients gave written informed consent before taking part in the study.
Ultrasound-guided needle biopsy
Percutaneous biopsies or transrectal biopsies were guided by transabdominal US or endorectal US, depending on the site of the tumors. Patients were fasted for 8–12 hours before the procedure. All examinations were performed using a Philips iU22 unit (Philips; Bothell, WA, USA). A convex array probe (C5-2) or an end-fire type endorectal probe (C5-9 sec) was utilized. B-mode and color Doppler US exams were preliminarily performed for all patients to choose the maximum solid area of the lesion or the rim parts of the large tumors as the optimal puncture site and to evaluate the safe needle pathway that could avoid vascular structures. The maximal length, echo pattern, and internal vascularity of the tumor were recorded. Patients underwent a cleansing enema before the transrectal ultrasonography and biopsy. The skin was sterilized, and local anesthetic was applied using 1% lidocaine before the percutaneous biopsies. Aiming at the previously determined optimal puncture site, an automatic biopsy gun (Bard Biopsy Systems; Tempe, AZ, USA) combined with an 18-gauge biopsy needle was used to obtain adequate tissue. The whole process was conducted under aseptic conditions. The specimens obtained were fixed and sent for histology and immuno-histochemistry. Patients were monitored for 3–4 hours after the procedure.
Contrast-enhanced ultrasound-guided needle biopsy
Percutaneous biopsies or and transrectal biopsies were guided by transabdominal or endorectal CEUS. Preparations were similar to those for the US-guided biopsies. The tumors were first evaluated using gray scale and Doppler US exams with a Philips iU22 unit (Philips; Bothell, WA, USA). A convex array probe (C5-2) or an end-fire type endorectal probe (C5-9 sec) were utilized. The mechanical index was 0.08–0.11. The focus point was just under the deep margin of the lesion. Thereafter, a 2.4-mL bolus of SonoVue® (Bracco, Italy) was intravenously injected in an antecubital vein, followed by a 5-mL flush with normal saline. The perfusion of the target lesion was continuously observed for at least 3 minutes. The area with the most pronounced contrast enhancement in the arterial phase without necrosis was determined as the target area. Then, a second 1.2 mL dose of SonoVue® was injected for real time guidance following a standardized procedure. An 18-gauge biopsy needle coupled on a BARD automatic biopsy gun was inserted in the targeted area. The specimens obtained were fixed and sent for histology and immuno-histochemistry. The patients were monitored for 3–4 hours after the procedure.
In both groups no patients had significant bleeding, pain, perforation or peritonitis developed after the biopsy.
Immunohistochemical staining using antibodies against CD34, CD117, S100, DOG1 and smooth muscle actin was performed in the specimens. When both CD117 and DOG1 were negative, GIST diagnosis was made when C-kit and/or platelet-derived growth factor receptor (PDGFR) were positive. Immunohistochemical diagnosis was based on recent guidelines (5-8,18-22).
Statistical analysis
Statistical analysis was performed using SPSS 19.9 (IBM Corp., Armonk, NY, USA). Categorical data are expressed as percentages, and continuous data are expressed as mean ± standard deviation. The difference in tumor size between the two protocols was analyzed using Mann-Whitney U test. Chi-squared test was performed to analyze qualitative parameters. Two-sided P values <0.05 were considered statistically significant.
Results
Tumor characteristics
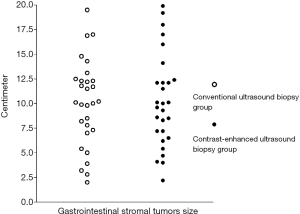
There were 14 lesions located in the stomach, the mean lesion size was 11.7 cm (range, 8.1–19.5 cm); 6 in the rectum, the mean lesion size was 6.7 cm (range, 2.0–10.0 cm); 4 in the duodenum, the mean lesion size was 13.8 cm (range, 8.5–19.0 cm); 3 in the liver, the mean lesion size was 4.2 cm (range, 2.2–5.4 cm), and 26 of uncertain origin, the mean lesion size was 10.1 cm (range, 6.2–19.9 cm). For all patients with uncertain origin surgery was not performed because the tumor was too large. Lesion size was not significantly different between the US and CEUS groups (Figure 1, 9.9±4.3 cm, n=30 vs. 10.2±4.6 cm, n=28; P=0.774).
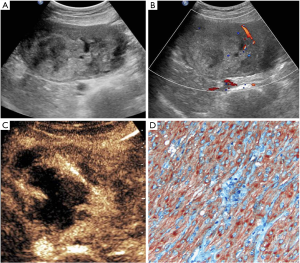
There were three lesions with homogenous enhancement and 25 lesions with heterogeneous enhancement in the CEUS group. Among the 25 lesions, 18 lesions show non-liquefied necrotic areas which appear hypoechoic or isoechoic on B-mode US, and a larger non-enhanced necrotic area than that detected as anechoic area by B-mode US was observed in 7 lesions (Figure 2). One case of rectal GIST had multiple small lesions (diameter 1.2–2.0 cm) located in distal rectum, presenting symptoms of perineal pain. Only one lesion in deeper muscular layer showed enhancement, while other lesions were not enhanced. In this case CEUS-guided biopsy for the target lesion acquired satisfactory specimen.
Diagnostic yield of ultrasound- and contrast-enhanced ultrasound-guided core needle biopsy
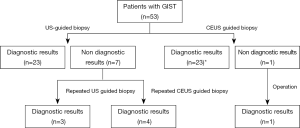
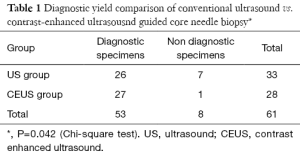
The biopsy working flow is shown in Figure 3. In the US group, there were 26 biopsy specimens with an accurate diagnosis of GIST. Three specimens showed only necrotic tissue, one specimen showed few atypical cells in large necrotic areas, one specimen revealed few spindle cells without cellular pleomorphism, and two specimens were suggestive of only a generic classification of mesenchymal tumors. The diagnostic yield of US guided biopsy for GIST was 83.3% (26/33). In the CEUS group, there were 27 biopsy specimens with accurate diagnosis of GIST. Only one specimen showed fibrous tissue and striated muscle without tumor cells. The diagnostic yield of CEUS guided biopsy for GIST was 96.2% (27/28). The difference in the diagnostic yield between the two groups was statistically significant (Table 1, JenyP=0.042).
Full table
A final diagnosis of gastrointestinal stromal tumors was made according to one of the following reference methods: (I) histological and immunohistochemical biopsy findings with definite proof of gastrointestinal stromal tumors in patients with unresectable tumours according to CT/MRI scan findings and compatible clinical follow-up (n=43). Thirty-six patients were diagnosed as GISTs at the first set of biopsy; 7 patients were diagnosed as GISTs with repeated biopsies; (II) ten patients accepted Imatinib as neoadjuvant therapy and underwent surgery eventually. All of the ten patients had definite histological diagnosis of gastrointestinal stromal tumors based on surgical resection specimens. CD 117(c-KIT) was positive in 49 patients, and 36 patients were positive for CD34. Fourteen patients had a molecular diagnosis based on an active mutation in c-KIT or/and active mutation of PDGFR.
The risk for undeterminable specimens was 27.3% (9/24 lesions) in the conventional US biopsy group, and 7.2% (2/26 lesions) in the CE US biopsy group (Table 2, JenyP=0.042). In both groups none patients had significant complications such as bleeding, pain, perforation or peritonitis after the biopsy.
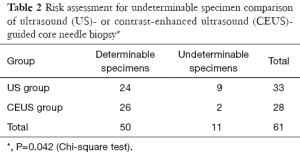
Full table
Discussion
It is well recognized that all GISTs have some degree of malignant potential. According to the tumor size, mitotic rate, and anatomic site, the risk is classified as very low-, low-, intermediate-, or high risk (23). Accurate preoperative diagnosis and risk stratification of GISTs is critical (24,25). For patients of GIST with metastatic disease or large locally advanced lesions, a biopsy is indicated to confirm the diagnosis before the initiation of tyrosine kinase inhibitor therapy. However, biopsy does not always provide sufficient material for an accurate histological diagnosis. Akahoshi et al. reported that tumor size is correlated with diagnostic yield and sensitivity (26). The diagnostic rate for the tumor less than 2, 2 to 4, and 4 cm or more were 71%, 86%, and 100%, respectively. In 29 surgically resected cases, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of endoscopic ultrasound guided final needle aspiration using immunohistochemical analysis of GIST were 100%, 80%, 96%, 100%, and 97%, respectively. Sepe et al. (27) reported the diagnostic yield and sensitivity of endoscopic ultrasound guided fine need aspiration cytology for the diagnosis of GIST was 78.4%. The sensitivity was 84.4% for GISTs located in the stomach, but poor for lesions located in the duodenum because none of these tumors yielded diagnostic cytology. The yield might actually decrease if the lesion is >10 cm, because larger tumors are more prone to necrosis (27). Another two studies showed an overall diagnostic accuracy for GIST of 84–92% with ultrasound fine needle aspiration (28,29). In cases with lesions for which endoscopic ultrasound fine needle aspiration is limited, percutaneous biopsy can be an effective alternative approach. Studies focusing on the diagnostic yield of percutaneous biopsies for GIST diagnosis are relatively lacking. Yeh et al. (11) compared 23 transluminal biopsies, 20 ultrasonography-guided biopsies, and 15 CT-guided biopsies; they reported failure rate was higher in the group of transluminal biopsies (17%).
In the present study, the diagnostic yield was 78.8% in the US group, which is similar to the results reported in the literature. The size of GISTs varied greatly, from a few millimeters to >30 cm, with a median size between 5 and 8 cm (mean: 10.1 cm). Gong et al. described majority of GIST are exophytic growth, necrosis is often seen in GIST and results in heterogeneous enhancement (30). Large GISTs might present with significant necrosis and cystic degeneration, with only a residual rim of viable tissue. The biopsy technique must be able to provide adequate and representative material to allow for a histopathological diagnosis. Sampling errors often happen if relying only on tissue texture during B-mode US. With contrast enhanced guided biopsy, hypervascular areas can be identified, and avascular and hypovascular areas such as necrosis, fibrosis, or desmoplastic tissue can be avoided. On the other hand, the non-liquefied necrotic area and hypovascular areas are difficult to identify on conventional grey scale US (31-35).
Our results confirmed CEUS guided biopsy improves the diagnostic yield and enables adequate sampling of GIST. In the present study, the proportions of biopsies with undeterminable samples were significantly different between the US group and CEUS group (9/33, 27.3% vs. 2/28, 7.2%, respectively), which can be attributed to more sufficient, representative, and viable tissue obtained with CEUS guided biopsy. GISTs rarely metastasize to the lymph node, while the liver is the most common metastatic site. In the present study, three biopsies of hepatic metastatic lesions were also obtained with diagnostic specimens.
Several studies have demonstrated US guided percutaneous core biopsy of gastrointestinal lesions is associated with a low rate of complications (11,36,37). In our series, we observed no immediate or delayed complications after the biopsy procedure during the follow-up. The results of this study suggest that, compared with conventional US guided core needle biopsy, CEUS guided core needle biopsy increases the diagnostic yield and may improves the risk assessment for the pre-treatment diagnosis of GIST. We recommend the inclusion of CEUS guided biopsy in the diagnostic work-up of advanced or metastatic GIST.
Acknowledgments
Funding: This study was supported by Beijing Municipal Science & Technology Commission (No. Z131107002213016); Beijing Hope Run Special Fund of China Cancer Research Foundation (CCRF) (No. LC2013A04); The Personnel Department of the People’s Republic of China Funds Preferred Activities of Science and Technology Project Funding; and PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (3320140103); Wu Jieping Medical Foundation (320675012622).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.04.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Cancer Hospital & Institute, Peking Union Medical College & Chinese Academy of Medical Sciences. The ID of the approval is NCC2013S-006. All patients gave written informed consent before taking part in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438:1-12. [Crossref] [PubMed]
- Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005;100:162-8. [Crossref] [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52-68. [Crossref] [PubMed]
- Wu JS, Goldsmith JD, Horwich PJ, et al. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology 2008;248:962-70. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Rubin BP, Fletcher JA, Fletcher CD. Molecular Insights into the Histogenesis and Pathogenesis of Gastrointestinal Stromal Tumors. Int J Surg Pathol 2000;8:5-10. [Crossref] [PubMed]
- Blackstein ME, Blay JY, Corless C, et al. Gastrointestinal stromal tumours: consensus statement on diagnosis and treatment. Can J Gastroenterol 2006;20:157-63. [Crossref] [PubMed]
- Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet 2007;369:1731-41. [Crossref] [PubMed]
- Tio TL, Tytgat GN, den Hartog Jager FC. Endoscopic ultrasonography for the evaluation of smooth muscle tumors in the upper gastrointestinal tract: an experience with 42 cases. Gastrointest Endosc 1990;36:342-50. [Crossref] [PubMed]
- Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy 2010;42:292-9. [Crossref] [PubMed]
- Yeh CH, Pan KT, Chu SY, et al. Safety and efficacy of image-guided percutaneous biopsies in the diagnosis of gastrointestinal stromal tumors. Clin Imaging 2012;36:19-23. [Crossref] [PubMed]
- Nesje LB, Laerum OD, Svanes K, et al. Subepithelial masses of the gastrointestinal tract evaluated by endoscopic ultrasonography. Eur J Ultrasound 2002;15:45-54. [Crossref] [PubMed]
- Spier BJ, Johnson EA, Gopal DV, et al. Predictors of malignancy and recommended follow-up in patients with negative endoscopic ultrasound-guided fine-needle aspiration of suspected pancreatic lesions. Can J Gastroenterol 2009;23:279-86. [Crossref] [PubMed]
- Xu X, Luo L, Chen J, et al. Acoustic radiation force impulse elastography for efficacy evaluation after hepatocellular carcinoma radiofrequency ablation: a comparative study with contrast-enhanced ultrasound. Biomed Res Int 2014;2014:901642.
- Kaneko OF, Willmann JK. Ultrasound for molecular imaging and therapy in cancer. Quant Imaging Med Surg 2012;2:87-97. [PubMed]
- Wáng YX, Choi Y, Chen Z, et al. Molecular imaging: from bench to clinic. Biomed Res Int 2014;2014:357258.
- Cui NY, Wang Y, Zhang R, et al. Contrast enhanced ultrasound demonstration of a proliferative mass in rectum wall in a patient after rectal cancer surgery. Quant Imaging Med Surg 2012;2:130-2. [PubMed]
- Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002;20:3898-905. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-4.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Medeiros F, Corless CL, Duensing A, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 2004;28:889-94. [Crossref] [PubMed]
- West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107-13. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Gheorghe M, Predescu D, Iosif C, et al. Clinical and therapeutic considerations of GIST. J Med Life 2014;7:139-49. [PubMed]
- Wang M, Xu J, Zhang Y, et al. Gastrointestinal stromal tumor: 15-years' experience in a single center. BMC Surg 2014;14:93. [Crossref] [PubMed]
- Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol 2007;13:2077-82. [Crossref] [PubMed]
- Sepe PS, Moparty B, Pitman MB, et al. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc 2009;70:254-61. [Crossref] [PubMed]
- Vander Noot MR 3rd, Eloubeidi MA, Chen VK, et al. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 2004;102:157-63. [Crossref] [PubMed]
- Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc 2009;69:1218-23. [Crossref] [PubMed]
- Gong J, Kang W, Zhu J, et al. CT and MR imaging of gastrointestinal stromal tumor of stomach: a pictorial review. Quant Imaging Med Surg 2012;2:274-9. [PubMed]
- Stock K, Hann von Weyhern C, Slotta-Huspenina J, et al. Microcirculation of subepithelial gastric tumors using contrast-enhanced ultrasound. Clin Hemorheol Microcirc 2010;45:225-32. [PubMed]
- Kitano M, Sakamoto H, Kudo M. Contrast-enhanced endoscopic ultrasound. Dig Endosc 2014;26:79-85. [Crossref] [PubMed]
- Yamashita Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J Clin Ultrasound 2015;43:89-97. [Crossref] [PubMed]
- Gong JS, Kang WY, Liu T, et al. CT findings of a gastrointestinal stromal tumor arising from small bowel. Quant Imaging Med Surg 2012;2:57-8. [PubMed]
- Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest Endosc 2011;73:227-37. [Crossref] [PubMed]
- Marco-Doménech SF, Gil-Sánchez S, Fernández-García P, et al. Sonographically guided percutaneous biopsy of gastrointestinal tract lesions. AJR Am J Roentgenol 2001;176:147-51. [Crossref] [PubMed]
- de Sio I, Funaro A, Vitale LM, et al. Ultrasound-guided percutaneous biopsy for diagnosis of gastrointestinal lesions. Dig Liver Dis 2013;45:816-9. [Crossref] [PubMed]


