
Clinical features and surgical outcomes of primary spinal anaplastic meningioma: a cases series and literature review
Highlight box
Key findings
• Primary spinal anaplastic meningioma (PSAM) is a rare disease. They may metastasize, recur, and portend a poor prognosis.
What is known and what is new?
• Seven PSAMs were reported in literature. Recurrence and metastasis were recorded though the data were usually incomplete.
• Three female and three male patients were diagnosed with PSAM, and the median age of the patients was 25 years old. After treatment, the median survival time was 14 months. Three had recurrence, two experienced metastases, and four died of respiratory failure.
What is the implication, and what should change now?
• PSAM is an extremely rare disease. The efficacy and safety of the treatment require further investigation. A close follow-up might be necessary.
Introduction
Meningiomas represent 40.0% of primary central nervous system (CNS) tumors, and 4.2% of meningiomas are located in spinal meninges (1). With a prevalence of 0.33 in 100,000 population, spinal meningiomas are usually benign and slow-growing tumors (2). However, primary spinal anaplastic meningioma (PSAM) is one of the rare malignant subtypes, which were pathologically classified as World Health Organization (WHO) grade III meningiomas (3-5). Distant metastasis could occur to intracranial meningiomas, and a systematic review concluded that 15.2% happened intraspinally (6). Accompanying metastasis from intracranial meningioma might complicate the clinical course, so we focus on PSAM, which developed and progressed originally within the spinal canal (6).
To the best of our knowledge, only seven articles concerning PSAM were published in English literature since Solero et al. reported the first case (7-13). Incomplete description was common, which increased the difficulty of understanding the disease (10,11). We noticed there were tumor recurrence, metastasis, and interestingly, “malignant transformation” recorded in literature (7-9). The phenomena seemed to be consistent with its WHO grading. We therefore report a case series of 6 patients with histologically confirmed PSAM at one single institution and discuss the clinical features, treatment strategy, and long-term outcomes of this rare entity. Hopefully, this will add to our knowledge of PSAM and provide raw data for further study. We present this article in accordance with the AME Case Series reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2505/rc).
Case presentation
Methods
From January 2008 to September 2017, six patients were consecutively admitted, surgically treated, and pathologically diagnosed with PSAM at the Department of Neurosurgery, Beijing Tiantan Hospital. All six patients were included based on the following criteria: (I) preoperative magnetic resonance imaging (MRI) demonstrated a lesion in the spinal canal; (II) intraoperative and histological findings confirmed the diagnosis of anaplastic meningioma; and (III) no other lesions in CNS or other systems were identified during initial diagnosis. We collected clinical data and completed follow-ups in December 2019 with the approval from institutional review board of Beijing Tiantan Hospital (No. KY-2019-076). The median follow-up time was 8.5 (range, 4–136) months. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case series and accompanying images was waived from patient consent according to the Beijing Tiantan Hospital ethics committee/institutional review board.
We routinely performed laminectomy and laminoplasty through posterior midline approach. Simpson grading was applied to define the extent of resection (14). The tumors which increased in size and caused relapsed or new neurological deficits after primary surgery were considered as tumor regrowth or recurrence. MRI with gadolinium contrast was routinely performed before and after surgery. Multimodal intraoperative neurophysiologic monitoring (IONM) was routinely applied to intra- and juxtamedullary tumors at our institution to identify and prevent potentially risky maneuvers (15,16).
Surgical specimens were all fixed in formaldehyde overnight and embedded in paraffin, which were analyzed through microscopic and immunohistochemical techniques and reviewed independently by two experienced neuropathologists. All pathological diagnoses met the criteria of anaplastic meningioma of WHO 2016 classification as below: (I) presence of at least 20 mitoses per 10 high-power fields, and/or (II) presence of overtly malignant morphological aspect resembling that of sarcomas, carcinomas or melanomas (5).
Modified McCormick classification (MMC) were applied to assess neurological function (17,18). This assessment was performed before surgery and during the last follow-up. All six patients or their kin consented to the use of their medical records for research purposes.
A comprehensive literature search was performed to identify articles relevant to PSAM. Keywords and MeSH terms such as “primary spinal anaplastic meningioma”, “spinal cord neoplasms” and “anaplastic meningioma” were incorporated into our search strategy. We used PubMed and the Ovid MEDLINE databases to identify the relevant English articles and found seven articles for inclusion (3,7-11).
Demographics and clinical presentations
Six PSAM patients at our center account for approximately 1% of all spinal meningiomas in the same period (Table 1). There were three male and three female patients with a median age of 25 (range, 13–46) years, and the duration of symptoms ranged from one week to one year. PSAMs occurred at cervical level in four patients, cervicothoracic in one, and thoracolumbar in one. Clinical presentations included focal pain, sensory deficits, and limb weakness, similar to that of common spinal tumors. The preoperative assessments showed that four patients were at MMC grade II (Case 1, 3, 4, 6), followed by one at grade Ib (Case 5). Case 2 was respectively classified as grade Ib and II during two different admissions.
Table 1
| Case | Age (years), sex | Duration from onset to diagnosis | Location | Presentations | MRI Findings | Treatment* | Modified McCormick | Metastasis | Follow-up | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1WI | T2WI | +GA | Pre-op | Follow-up | ||||||||
| 1 | 20, male | 1.5 months | C1-2, IM & IDEM | Right side weakness and reduced superficial sensation | Iso | Mixed hyper | Hetero | IV + RT | II | NA | Disseminated to thoracic spinal canal | 9 months, rec†; 20 months, dead† |
| 2 | 46, male | 4 months | C1-3 (1st), C1-2 (2nd), IDEM | Neck pain and head tightness; right upper limb weakness (2nd) | Mixed iso | Mixed hyper | Hetero | IV + RT (1st); II (2nd) | Ib (1st) | I (1st) | Non (1st) | 11 years, rec; 11 years & 4 months, alive |
| II (2nd) | I (2nd) | Non (2nd) | ||||||||||
| 3 | 19, male | 1 year | C6-T1, IDEM | Left scapula pain, hands atrophy& weakness | Iso | Mixed hyper | Hetero | V + IV + RT | II | NA | Brain, femurs and multiple areas | 8 months, rec & dead |
| 4 | 46, female | 1 week | C3-4, IDEM | Left upper arm weakness | Iso | Hyper | Homo | II + RT | II | NA | NA | 4 months, no rec & dead |
| 5 | 30, female | 6 months | T12-L1, IDEM | Lower back pain and reduced superficial sensation | Iso | Hyper | Homo | II + RT | Ib | I | Non | 9 years, no rec & alive |
| 6 | 13, female | 1 month | FM/C1-C2, IDEM | Neck & head pain and left side weakness | Iso | Hyper | Homo | II | II | NA | NA | 4 months, no rec & dead |
*, extent of resection was recorded according to Simpson (I–V) resection; †, since initial diagnosis. MRI, magnetic resonance imaging; T1WI, T1 weighted imaging; T2WI, T2 weighted imaging; +GA, gadolinium administration; IM, intramedullary; IDEM, intradural and extramedullary; Iso, isointensity; Hyper, hyperintensity; Hetero, heterogeneously; RT, radiotherapy; NA, not available; Rec, recurrence; FM, foramen magnum; Homo, homogeneously.
Radiological findings
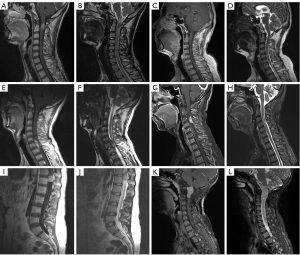
Six PSAM cases generally demonstrated isointensity on T1WI, hyperintensity on T2WI, and hetero- (Case 1–3) or homogeneously (Case 4–6) marked enhancement with contrast (Figure 1). Two of the lesions were spindle-shaped (Case 2, 3), two were lobular (Case 1, 6) and two were spherical (Case 4, 5). All tumors were located at intradural and extramedullary space, while spinal cord was infiltrated in Case 1. Three PSAMs grew on the ventral side of the spinal cord (Case 2, 3, 6), while three on lateral side (Case 1, 4, 5). Case 1, 4, and 6 presented hyperintensity of the spinal cord on T2WI to certain extent.
Pathological examination
Histologically, markedly elevated mitosis, abundant cytoplasm, prominent nucleoli and extensive necrosis could be found, which met the criteria of the WHO 2016 classification of anaplastic meningioma (Figure 2). Immunohistochemical analyses were also performed, reporting high Ki-67 indices as well as positive Vimentin and epithelial membrane antigen (EMA) in all cases. Ki-67 indices were 20%, 40%, 30%, 40%, 60%, 30%, respectively (Case 1–6).

Treatment
Eight operations were performed in six patients. Simpson grade II resection was achieved in four (50%, Case 2, 4–6), grade IV in three (37.5%, Case 1–3), grade V in one (12.5%, Case 3). Intraoperatively, we observed tumors with various textures, ranging from elastic to firm, and found irregularly brownish surfaces with well-defined capsules. PSAM in Case 1 had infiltration into spinal cord at the upper side of the tumor. IONM reported no abnormalities in all six patients during surgery. Postoperative courses were uneventful in five patients, and they were therefore discharged at 10th day after surgery. Case 4 was put on ventilator for 15 days and transferred to a local hospital. Adjuvant radiotherapy was recommended after surgery. However, one patient refused the treatment (Case 6), and one patient chose to wait and see, because he had radiotherapy 11 years ago (Case 2).
Long-term outcomes
We completed follow-ups in December 2019 and found the median survival time after the initial diagnosis was 14 (range, 4–136) months. Three patients had recurrence (Case 1–3), two experienced metastases (Case 1, 3) and four died (Case 1, 3, 4 ,6) at the completion of the last follow-ups. The median time for recurrence was 9 (range, 8–11) months. PSAM in Case 3 metastasized to brain, femur and multiple other sites, while PSAM in Case 1 recurred and disseminated to thoracic canal. All four deceased patients died of respiratory failure with lung infection due to confinement to bed. Case 2 survived over 11 years since initial diagnosis, and Case 5 survived 9 years without major complications. They both were clinically and radiologically stable without evidence of tumor regrowth and returned to MMC grade I status.
Illustrative cases
Case 2
A 46-year-old man complaint of intermittent neck pain and head tightness for 4 months and claimed it could be relieved by acupuncture. No neurological deficit was found. The MRI scan showed a lesion with mixed isointensity on T1WI, mixed hyperintensity on T2WI, and heterogeneously marked enhancement with contrast at C1-3 level. He underwent subtotal resection of the ventral tumor (Simpson IV resection), and a PSAM was pathologically confirmed. Postoperatively, the previous symptoms were all relieved and the MRI demonstrated the ventral residue. The patient subsequently underwent adjuvant radiotherapy at a local hospital. Eleven years later, he was again admitted with progressive right upper limb weakness. A muscle strength of grade 4/5 was observed in the whole right upper limb, and no other abnormality was found. MRI demonstrated homogeneously marked enhancement at C1-2 level, which indicated tumor recurrence. The patient achieved gross total resection (Simpson II resection) this time and refused adjuvant radiotherapy. The postoperative course was uneventful, the limb weakness was significantly improved, and postoperative MRI proved no residue. Four months later, no recurrence or metastasis was found.
Case 3
A 19-year-old man presented with progressive left scapula pain for approximately 1 year, and muscle atrophy of both hands was observed. MRI demonstrated heterogeneously marked enhancement at C6-T1. He underwent biopsy initially at another hospital which confirmed a PSAM. We admitted him, performed subtotal resection of the ventral tumor (Simpson IV resection), and confirmed the diagnosis. Radiotherapy was arranged afterwards. He regained ambulation without cane but soon found a growing mass beneath his laryngeal prominence. Therefore, a positron emission tomography scan was arranged at a local hospital and reported metastases to brain, femurs and multiple areas. Seven months later, he suffered quadriplegia with pain all over the body. Soon, he died of respiratory failure with lung infection.
Discussion
Epidemiology and clinical features
PSAM is extremely understudied due to its rarity (Table 2). Spinal meningioma is generally considered to have female predilection and the corresponding male to female ratio ranges from 1:1.5 to 1:14.5 (2,19,20). However, the numbers of male and female patients are even in our small sample. The incidence of meningioma increases over age, and people aged over 65 are most commonly affected (21). Similarly, spinal meningiomas most frequently occur in older patients (22). A systematic review concluded the average age of spinal meningiomas was 62.6 years old after adjusting the sample size of various studies (20). The median age of our patients was 25 (range, 13–46) years, while the median age of literature cases was 55 (range, 47–68) years (8,9,12,13). Both samples were small and apparently heterogenous, thereby offering no consistent information concerning susceptible age. A case series reported 73% of spinal meningiomas were located in the thoracic region, 16% in cervical, and 4.5% at thoracolumbar junction (23). However, PSAMs seemed to occur more frequently in cervical region (66.7%) followed by cervicothoracic (16.7%) and thoracolumbar areas (16.7%) in our sample. In literature, four PSAMs were all found at thoracic level (7-9,12).
Table 2
| Authors & years | Cases | Age (years), sex | Location | Presentations | Recurrence* | Metastasis | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Solero et al., 1989 (7) | 1 | NA | T4-5; IDEM | Slight neurologic deficit | 9 years | NA | GTR (1st); STR (2nd) | 10 years, dead* |
| Nishida et al., 2016 (8) | 1 | 68, male | C7-T2; IM & IDEM | Back pain and left lower limb paralysis | Non | Mediastinal lymph nodes | STR + RT | NA |
| Ye et al., 2016 (10) | 2 | NA | NA | NA | Within 5 years | NA | NA | Within 5 years, dead |
| NA | NA | NA | Non | NA | NA | 5 years, alive* | ||
| Ito et al., 2017 (9) | 1 | 60, male | T2-3; intra- & extraspinal | Thoracic pain | Non | Rib, lumbar vertebra, and sacrum | Biopsy | 1 year, dead |
| Hua et al., 2018 (11) | 8 | NA | IDEM | NA | NA | NA | NA | NA |
| Ampie et al., 2021 (12) | 1 | 50, male | Thoracic, IM & IDEM | NA | NA | NA | STR + RT + Sunitinib | 1 month, dead |
| Krauss et al. 2021 (13) | 1 | 47, female | NA | Neck/back and extremity pain; sensory deficit | Non | NA | STR + RT | Dead |
*, since initial diagnosis. NA, not available; IDEM, intradural and extramedullary; GTR, gross-total resection; STR, subtotal resection; IM, intramedullary; RT, radiotherapy.
Pain and limb weakness might be the most common initial symptoms, leading to the difficulty of differentiating PSAMs from other spinal tumors. The duration of symptoms varied from 1 week to 6 months, and MMC grading varied from Ib to II. The literature cases revealed similar clinical presentations, though duration of symptoms and MMC grading were hardly recorded (7-13).
The radiological features of PSAM were rarely mentioned in literature. Ito et al. and Nishida et al. respectively reported a case with homogeneous enhancement and swelling spinal cord (8,9). PSAMs in our study demonstrated a relatively consistent presentation. A study found non-spherical shape as well as the intratumoral heterogeneity on MRI had a significant association with high-grade intracranial meningiomas (WHO II–III) (24). In our sample, PSAMs presented spindle-shaped or lobular in four (66.7%), spherical in two (33.3%) and showed heterogeneity with contrast in three patients. In addition, the spinal cord swelling occurred in three (50%) patients. Ito et al. found PSAM could surround the spinal cord, occupy the limited intradural extramedullary space, and cause the swelling of the cord (9). Nishida et al. recorded the spindle-shaped PSAM grew on the left side and also caused the swelling (8). Further study on the correlation between the tumor morphology and intratumoral heterogeneity might assist in differential diagnosis of spinal meningiomas of different WHO grading.
Histopathology
The fifth edition of WHO grading of CNS tumors focuses on the integrated diagnosis of histology, immunohistochemistry, and molecular diagnostics (25). For meningiomas, anaplasia still features in WHO grade III tumors (25). Malignant features such as prominent nucleoli, extensive necrosis and markedly elevated mitosis might all explain the aggressive biological behaviors of anaplastic meningiomas (5). However, anaplasia in spinal meningiomas was rarely reported (7,10-13).
Molecular diagnostics and immunohistochemistry are two critical tools for classification, though we know little about the overlap between them and anaplasia. Li et al. pointed that Ki-67 index might serve as an indicator for poor prognosis in meningiomas (26). We routinely performed the Ki-67 staining and found the median index was 35% (range, 20–60%), which indicated highly proliferative growth of PSAM. However, we could not conclude anything confirmative about the index and patient’s clinical outcomes. We know the presence of telomerase reverse transcriptase (TERT) promotor mutation or homozygous loss of CDKN2A/B could also define WHO grade III meningioma (25). TERT mutation correlates with higher risk of recurrence and mortality in meningiomas (27). Mirian et al. therefore suggested aggressive surveillance of TERT genetic alterations (27). CDKN2A/B homozygous deletion was another factor associated with early recurrence and highly prognostic in meningiomas, whose monitoring was also suggested by Sievers et al. (28). Unfortunately, our study was retrospective, and the genetic sequencing was not performed. Therefore, we failed to find any association between the genetic alterations and anaplasia in PSAM.
Interestingly, Ito et al. reported the malignant transformation of spinal atypical meningiomas to PSAM (9). Though we did not find similar phenomena, this was recorded in intracranial anaplastic meningiomas, revealing histological heterogeneity as well as malignant progression from low-grade ones. The mechanisms remain poorly understood. However, this might warn us of thorough survey of the tumor pathology.
Treatment and outcomes
National Comprehensive Cancer Network recommend complete surgical resection to symptomatic meningiomas (if feasible) and adjuvant radiotherapy if the meningioma was pathologically defined WHO grade III tumor (29). In addition, systematic therapies might be considered once the tumor was not amenable to surgery or radiation (29). However, PSAM is located at spinal canal, which might affect the treatment of choice, and the clinical outcomes remain unclear. Furthermore, no standard protocol might be established due to the rarity, and thereby case series is a critical reference for future cases.
Surgery is the first consideration for treatment and pathological classification (10,11,13,29,30). Gross total resection should be the best line of treatment whenever feasible (13,29). However, Simpson grade I resection in spinal canal is usually difficult to achieve due to limited space and we performed Simpson grade II resection in 50% operations. A systematic review investigated 13 studies regarding spinal meningiomas and found there was no significance between Simpson grade I and II with respect to tumor recurrence (30). In addition, higher Simpson grade of resection could significantly increase recurrence over time (30,31). Therefore, a more comprehensive treatment option should be trialed.
The role of adjuvant radiotherapy in treating PSAM is rather unclear. Five patients in our sample applied radiotherapy, and the outcomes were different. Due to lack of study in PSAM, we could only reference the trial or experience from intracranial anaplastic meningiomas. A study based on National Cancer Database observed a survival benefit for performing both gross total resection and radiotherapy (32). However, another retrospective cohort study of intracranial anaplastic meningioma suggested though conventional adjuvant radiotherapy helped local tumor control, its effects on overall survival remained controversial (33). The initial results of phase II RTOG 0539 trial revealed that the 3-year local control and overall survival rate of the patients with high-risk meningioma (including new anaplastic meningiomas) treated with intensity-modulated radiotherapy was 68.9% and 78.6%, respectively (34). The authors believed the results favored postoperative intensity-modulated radiotherapy.
No standard chemotherapy concerning anaplastic meningioma treatment has been established yet (29). However, multiple drugs have been on certain experimental therapy or early-stage clinical trials (35,36). Among them, bevacizumab, an anti-angiogenic drug, was found to have induced partial response in an experimental therapy of a patient with intracranial anaplastic meningioma (35). In addition, Sunitinib was proven active in treating recurrent malignant meningiomas despite its toxicity in a phase II trial.
In our sample, PSAMs had poor outcomes overall with a median survival time of 14 months after initial diagnosis. Recurrence occurred to three patients, and the recurrence-free survival time ranged from eight months to eleven years. Among the three, two were severely affected by multiple metastases and thus refused further treatment. The two patients died consequently. Two other patients without recurrence both died at 4-month after initial diagnosis. The respiratory failure induced by lung infection was the mortality cause. El-Hajj et al. concluded that the recurrence rate of spinal meningiomas was 6%, and the higher WHO grade, higher Simpson grade, ventral location, and male sex were all correlated with higher recurrence (30). By contrast, the recurrence rate of PSAM reached 50% in our sample and 33.3% (2/6) in literature, which was consistent with El-Hajj’s finding. Interestingly, all our male patients experienced recurrence while females did not. In both our sample and literature, metastasis was another dismal event. Nishida et al. reported that their patient experienced metastasis to mediastinal lymph nodes, while Ito et al. recorded PSAM metastasis to rib, lumbar vertebra, and sacrum (8,9). The former patient’s survival status was not available, and the latter died one year after initial diagnosis.
In literature, the survival time was heterogenous, ranging from 1 month to 10 years (7-13). Despite the unfavorable prognosis, two patients of ours respectively survived about eleven years and nine years without major complications and achieved favorable recovery. Though Case 2 had recurrence 11 years after Simpson IV resection followed by radiotherapy, he witnessed no regrowth 4 months after another Simpson II resection. We could not conclude anything confirmative about the survival, because there was no significance regarding gender, extent of resection, or even Ki-67 indices (40% for Case 2; 60% for Case 5). However, both of them presented with MMC grade Ib at initial diagnosis, and no metastasis happened to either of them. Some bold speculations might be that the timing of intervention and certain tumor statuses somehow spared the patients. Though the two survivors were alive and classified as MMC grade I after some years’ follow-up, regular checks will still be needed.
Limitations
There are a few limitations about our retrospective study. Firstly, there might be recall bias, because the last follow-up was done through telephone if the patient was deceased. Secondly, we did not perform genetic analysis, which hardly explained the potential relationship on molecular levels. Thirdly, we could only apply the best treatment plan at the time. Lastly, due to the limited sample size, we could not conclude anything confirmative.
Conclusions
PSAMs are a rare disease, and there is limited evidence as to the management of these lesions. They may metastasize, recur, and portend a poor prognosis. A close follow-up and further investigation are therefore necessary.
Acknowledgments
We would like to thank all the patients who trusted us and all the physicians and staff who helped in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2505/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2505/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2505/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by institutional ethics committee of Beijing Tiantan Hospital (No. KY-2019-076). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case series and accompanying images was waived from patient consent according to the Beijing Tiantan Hospital ethics committee/institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ostrom QT, Price M, Neff C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019. Neuro Oncol 2022;24:v1-95. [Crossref] [PubMed]
- Kshettry VR, Hsieh JK, Ostrom QT, et al. Descriptive Epidemiology of Spinal Meningiomas in the United States. Spine (Phila Pa 1976) 2015;40:E886-9. [Crossref] [PubMed]
- Sade B, Chahlavi A, Krishnaney A, et al. World Health Organization Grades II and III meningiomas are rare in the cranial base and spine. Neurosurgery 2007;61:1194-8; discussion 1198. [Crossref] [PubMed]
- Wright JM, Wright CH, Cioffi G, et al. Survival in Patients with High-Grade Spinal Meningioma: An Analysis of the National Cancer Database. World Neurosurg 2019;129:e749-53. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. editors. WHO Classification of Tumours of the Central Nervous System (Revised 4th edition). 2016.
- Surov A, Gottschling S, Bolz J, et al. Distant metastases in meningioma: an underestimated problem. J Neurooncol 2013;112:323-7. [Crossref] [PubMed]
- Solero CL, Fornari M, Giombini S, et al. Spinal meningiomas: review of 174 operated cases. Neurosurgery 1989;25:153-60. [Crossref] [PubMed]
- Nishida N, Kanchiku T, Imajo Y, et al. A case of an anaplastic meningioma metastasizing to the mediastinal lymph nodes. J Spinal Cord Med 2016;39:484-92. [Crossref] [PubMed]
- Ito K, Imagama S, Ando K, et al. Intraspinal meningioma with malignant transformation and distant metastasis. Nagoya J Med Sci 2017;79:97-102. [PubMed]
- Ye J, Lv G, Qian J, et al. Clinical features and prognostic factors of WHO II and III adult spinal meningiomas: analysis of 25 cases in a single center. J Neurooncol 2016;128:349-56. [Crossref] [PubMed]
- Hua L, Zhu H, Deng J, et al. Clinical and prognostic features of spinal meningioma: a thorough analysis from a single neurosurgical center. J Neurooncol 2018;140:639-47. [Crossref] [PubMed]
- Ampie L, Snyder MH, Dominguez JF, et al. Clinical characteristics and long-term outcomes for patients who undergo cytoreductive surgery for thoracic meningiomas: a retrospective analysis. Neurosurg Focus 2021;50:E18. [Crossref] [PubMed]
- Krauss WE, Yolcu YU, Alvi MA, et al. Clinical characteristics and management differences for grade II and III spinal meningiomas. J Neurooncol 2021;153:313-20. [Crossref] [PubMed]
- Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22-39. [Crossref] [PubMed]
- Daniel JW, Botelho RV, Milano JB, et al. Intraoperative Neurophysiological Monitoring in Spine Surgery: A Systematic Review and Meta-Analysis. Spine (Phila Pa 1976) 2018;43:1154-60. [Crossref] [PubMed]
- Sutter M, Eggspuehler A, Jeszenszky D, et al. The impact and value of uni- and multimodal intraoperative neurophysiological monitoring (IONM) on neurological complications during spine surgery: a prospective study of 2728 patients. Eur Spine J 2019;28:599-610. [Crossref] [PubMed]
- McCormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg 1990;72:523-32. [Crossref] [PubMed]
- Aghakhani N, David P, Parker F, et al. Intramedullary spinal ependymomas: analysis of a consecutive series of 82 adult cases with particular attention to patients with no preoperative neurological deficit. Neurosurgery 2008;62:1279-85. [PubMed]
- Westwick HJ, Shamji MF. Effects of sex on the incidence and prognosis of spinal meningiomas: a Surveillance, Epidemiology, and End Results study. J Neurosurg Spine 2015;23:368-73. [Crossref] [PubMed]
- El-Hajj VG, Pettersson-Segerlind J, Fletcher-Sandersjöö A, et al. Current Knowledge on Spinal Meningiomas Epidemiology, Tumor Characteristics and Non-Surgical Treatment Options: A Systematic Review and Pooled Analysis (Part 1). Cancers (Basel) 2022;14:6251. [Crossref] [PubMed]
- Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol 2019;21:v1-100. [Crossref] [PubMed]
- Cohen-Gadol AA, Zikel OM, Koch CA, et al. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg 2003;98:258-63. [PubMed]
- Sandalcioglu IE, Hunold A, Müller O, et al. Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J 2008;17:1035-41. [Crossref] [PubMed]
- Coroller TP, Bi WL, Huynh E, et al. Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One 2017;12:e0187908. [Crossref] [PubMed]
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23:1231-51. [Crossref] [PubMed]
- Li J, Liang R, Song C, et al. Prognostic Value of Ki-67/MIB-1 Expression in Meningioma Patients: A Meta-Analysis. Crit Rev Eukaryot Gene Expr 2019;29:141-50. [Crossref] [PubMed]
- Mirian C, Duun-Henriksen AK, Juratli T, et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry 2020;91:378-87. [Crossref] [PubMed]
- Sievers P, Hielscher T, Schrimpf D, et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol 2020;140:409-13. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Central Nervous System Cancers (Version 2.2022). 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- El-Hajj VG, Pettersson-Segerlind J, Fletcher-Sandersjöö A, et al. Current Knowledge on Spinal Meningiomas-Surgical Treatment, Complications, and Outcomes: A Systematic Review and Meta-Analysis (Part 2). Cancers (Basel) 2022;14:6221. [Crossref] [PubMed]
- Aizer AA, Bi WL, Kandola MS, et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer 2015;121:4376-81. [Crossref] [PubMed]
- Orton A, Frandsen J, Jensen R, et al. Anaplastic meningioma: an analysis of the National Cancer Database from 2004 to 2012. J Neurosurg 2018;128:1684-9. [Crossref] [PubMed]
- Alhourani A, Aljuboori Z, Yusuf M, et al. Management trends for anaplastic meningioma with adjuvant radiotherapy and predictors of long-term survival. Neurosurg Focus 2019;46:E4. [Crossref] [PubMed]
- Rogers CL, Won M, Vogelbaum MA, et al. High-risk Meningioma: Initial Outcomes From NRG Oncology/RTOG 0539. Int J Radiat Oncol Biol Phys 2020;106:790-9. [Crossref] [PubMed]
- Puchner MJA, Hans VH, Harati A, et al. Bevacizumab-induced regression of anaplastic meningioma. Ann Oncol 2010;21:2445-6. [Crossref] [PubMed]
- Kaley TJ, Wen P, Schiff D, et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol 2015;17:116-21. [Crossref] [PubMed]


