
Prognostic value of prior malignancy history in stage I differentiated thyroid cancer: a SEER-based study
Highlight box
Key findings
• Prior malignancy history has an adverse impact on the survival of patients with stage I DTC. The probability of achieving 5-year overall survival for stage I DTC patients with prior malignancy history increases with each additional year survived.
What is known and what is new?
• Some clinicopathologic factors associated with the prognosis of thyroid cancer;
• After considering the competitive risks, prior malignancy history was a risk factor for the DTC-related deaths.
What is the implication, and what should change now?
• The inconsistent survival effects of prior malignancy history should be considered in clinical trial design and recruitment of stage I DTC patients.
Introduction
Thyroid cancer is the most common endocrine cancer today (1). In 2020, 586,202 new cases and 43,646 deaths were reported due to thyroid cancer worldwide. Thyroid cancer is the fifth most common malignancy for women worldwide that year (2). Differentiated thyroid cancer (DTC) comprises more than 95% of all thyroid cancers (1). Four types of DTC are recorded: the most frequent forms are papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), with PTC accounts for more than 85% of all DTC cases. The third and fourth types of DTC are Hürthle cell cancer and poorly DTC (3).
The risk of thyroid cancer-related death at 10 years for stage I DTC based on the American Joint Committee on Cancer (AJCC) 7th edition and 8th edition staging system are both less than 2% (3). Compared with the 7th edition, one-third of patients are downstaged in the 8th edition, and more than 90% of patients have stage I or II DTC (3); in some studies, the 10-year disease-specific survival (DSS) for stage I DTC based on the AJCC 7th edition reached 100% (4). Assessment of the risk of recurrence and progression is a dynamic process. The risk of recurrence and disease-specific mortality can change over time; therefore, recurrence risk estimates should be continually modified during follow-up (5).
With recent progress in the screening, diagnosis, and treatment of many cancers, patients with early-stage cancer are more likely to be detected, cancer mortality is reduced, and survival time is much longer (2,6-10). In this case, patients with a history of cancer may suffer from a second primary cancer or multiple primary cancers, including thyroid cancers, in the post-treatment follow-up. Rosso et al. reported that in different European countries, multiple primary tumors account for an overall proportion of 6.3% of all considered cancers and range from 0.4% to 12.9% in different countries. For cancers with high incidence and long survival, the proportion of multiple tumors is higher (11). Bhatti et al. reported a 29% increased risk of thyroid cancer after any primary malignancy, when compared to the general population (12). Lal et al. found that 12 months after the initial diagnosis of most common form of invasive cancer, standardized incidence ratios for subsequent primary thyroid cancer were greatly elevated especially after the account of renal and female breast cancers in nonirradiated patients (13).
The Surveillance, Epidemiology, and End Results (SEER) Program provides information on cancer statistics among the U.S. population, and SEER is an authoritative source for cancer statistics in the United States. The SEER database consists of 18 cancer registries. Although this public database provides relatively comprehensive information about tumor clinical characteristics and pathologic feature, it also has some limitations. For example, the lack of some important information and detailed treatment options.
To date, most studies regarding DTC are based on data from single-center studies or multicenter studies and have focused on the clinical characteristics and pathologic features that influence prognosis (14,15). Few studies based on SEER have evaluated the relationship of a prior malignancy history and the prognosis of thyroid cancer (13,16).
In this study, we surveyed the impact of a prior malignancy history on the prognosis of individuals with DTC based on SEER, to confirm the prognostic value of a prior malignancy history and provide evidence for the development of follow-up strategies for specific DTC patients. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2232/rc).
Methods
Patient selection
The SEER database (https://seer.cancer.gov/) was employed in the present study. In this study, SEER*Stat software (version 8.3.9) was used to obtain access. The “Incidence-SEER 18 Regs Custom Data (with additional treatment field), Nov 2018 Sub (1975‐2016 varying)” database was chosen in this study. The data from SEER are public and do not involve the privacy of patients; therefore, review and consent of the ethics committee are not needed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
A total of 210,045 thyroid cancer patients were identified for the initial analysis. Next, patients with histology codes of 8050 (papillary carcinoma, NOS), 8260 (papillary adenocarcinoma, NOS), 8330–8332 (follicular adenocarcinoma), and 8340–8344 (papillary carcinoma_follicular varian/microcarcinoma/oxyphilic cell/encapsulated/columnar cell) were included (NOS means nothing otherwise special). All patients with AJCC stage I were included (AJCC 7th edition). Patients whose “Survival months” were “0” and “Not available” were excluded. Finally, 49,723 eligible patients remained. Information about patient demographics and cancer characteristics, such as sex, age, year of diagnosis, race, marital status, tumor grade, T stage, N stage, histological type, treatment, prior malignancy history and survival time were obtained.
Study variables
The definition and information regarding the variables of sex, diagnosis age, year of diagnosis, race, marital status, tumor grade, histological type, and survival time can be found in the SEER database. Overall survival (OS) and disease‐specific survival (DSS) were the primary study endpoints. For OS (variable name: outcome), patients were divided into two groups: deceased (value: 1) and alive (value: 0). For DSS (variable name: outcome2), patients were divided into two groups: those who died of DTC (value: 1) and others (value: 0). To perform the competing risk model, a new variable was defined (variable name: outcome3), and patients were divided into three groups: those who died of DTC (value: 1); those who died of other causes (value: 2) and those who survived (value: 0). For age, patients were divided into three groups (variable name: Age.cat): ≤40, 41–60, and ≥61 years old. For race, patients were divided into two groups: white and others. For marital status, patients were divided into three groups: a single or unmarried group, a married group, and others. For the diagnosis year, all patients were divided into two groups: diagnosed between 2011–2012 and 2013–2015. For AJCC T stage (variable name: AJCC.T), patients were divided into two groups: T0–T2/Tx and T3–T4. The AJCC N stage (variable name: AJCC.N) was also divided into two groups: N0/Nx and N1. For the tumor size (variable name: Tumor.size), patients were divided into three groups: ≤1, 1–2 and >2 cm. For the tumor grade, patients were divided into three groups: I, II–III and unknown. For the histological types (variable name: Hist.type), Patients were divided into two groups: PTC (PTC_NOS) and others. For the surgery method (variable name: Surgery.method), patients were divided into four groups: subtotal thyroidectomy, total thyroidectomy, none and others. Patients were divided into two groups based on whether they underwent radiotherapy: those who underwent radiotherapy (Yes) and those who did not undergo radiotherapy or were unknown (No/Unknown). Patients were also divided into two groups based on whether they underwent chemotherapy: those who underwent chemotherapy (Yes) and those who did not undergo chemotherapy or were unknown (No). Additionally, patients were divided into two groups based on whether or not they had a prior malignancy or malignancies (Is.primary): without prior malignancy (Yes) and with prior malignancy or malignancies (No).
Statistical analysis
R (version 4.0.5) was used for the statistical analysis and visualization. Normality was tested using the Shapiro-Wilk normality test. Comparisons between two groups of continuous variables were performed using the Wilcoxon rank-sum test or two-sample t-test depending on normality. Comparisons between two or more groups of count variables were performed using the chi-squared test. Some R packages were used in this study. The “survival” package was used for the Kaplan-Meier survival analysis and Cox regression analysis; the “tidyverse” package was also used for the conditional survival analysis; the “forestplot” package was used for the drawing of forest plots; and the “cmprsk” package was used for competing risk model building. A P value of less than 0.05 defined statistical significance.
Results
The baseline demographic and clinicopathological characteristics of the enrolled patients in the SEER database
Finally, 49,723 stage I DTC patients were enrolled in our study. Among them, 4,982 had prior malignancy history (“Is.primary”: No), and 44,741 had no prior malignancy history (“Is.primary”: Yes). The patients’ baseline demographic characteristics and clinicopathological characteristics are summarized in Table 1. Comparisons between groups showed significant differences in some variables, including “Age”, “Age.cat”, “Sex”, “Race”, “Marital”, “AJCC.T”, “AJCC.N”, “Tumor.size”, “Hist.type”, “Surgery.method”, “Radiotherapy”, “Chemotherapy”, “Survival.months”, “Outcome”, “Outcome2”, and “Outcome3” (all P<0.05).
Table 1
| Variables | Total (n=49,723) | Is.primary | P value | |
|---|---|---|---|---|
| No (n=4,982) | Yes (n=44,741) | |||
| Age (years), median [Q1, Q3] | 44 [35, 57] | 59 [46, 68] | 43 [34, 55] | <0.001 |
| Age.cat, n (%) | <0.001 | |||
| ≤40 years | 20,158 (40.54) | 741 (14.87) | 19,417 (43.4) | |
| 41–60 years | 19,954 (40.13) | 1,966 (39.46) | 17,988 (40.2) | |
| ≥61 years | 9,611 (19.33) | 2,275 (45.66) | 7,336 (16.4) | |
| Sex, n (%) | <0.001 | |||
| Female | 40,118 (80.68) | 3,620 (72.66) | 36,498 (81.58) | |
| Male | 9,605 (19.32) | 1,362 (27.34) | 8,243 (18.42) | |
| Race, n (%) | <0.001 | |||
| Others | 9,600 (19.31) | 746 (14.97) | 8,854 (19.79) | |
| White | 40,123 (80.69) | 4,236 (85.03) | 35,887 (80.21) | |
| Marital, n (%) | <0.001 | |||
| Married | 29,191 (58.71) | 2,903 (58.27) | 26,288 (58.76) | |
| Others | 8,480 (17.05) | 1,209 (24.27) | 7,271 (16.25) | |
| Single/unmarried | 12,052 (24.24) | 870 (17.46) | 11,182 (24.99) | |
| Diagnosis, n (%) | 0.098 | |||
| 2011–2012 | 23,852 (47.97) | 2,334 (46.85) | 21,518 (48.09) | |
| 2013–2015 | 25,871 (52.03) | 2,648 (53.15) | 23,223 (51.91) | |
| AJCC.T, n (%) | <0.001 | |||
| T0–T2/Tx | 44,029 (88.55) | 4,756 (95.46) | 39,273 (87.78) | |
| T3–T4 | 5,694 (11.45) | 226 (4.54) | 5,468 (12.22) | |
| AJCC.N, n (%) | <0.001 | |||
| N0/Nx | 41,764 (83.99) | 4,669 (93.72) | 37,095 (82.91) | |
| N1 | 7,959 (16.01) | 313 (6.28) | 7,646 (17.09) | |
| Tumor.size, n (%) | <0.001 | |||
| 1–2 cm | 15,148 (30.46) | 1,575 (31.61) | 13,573 (30.34) | |
| ≤1 cm | 24,369 (49.01) | 3,006 (60.34) | 21,363 (47.75) | |
| >2 cm | 10,206 (20.53) | 401 (8.05) | 9,805 (21.92) | |
| Grade, n (%) | 0.056 | |||
| I | 10,851 (21.82) | 1,038 (20.84) | 9,813 (21.93) | |
| II–III | 1,804 (3.63) | 163 (3.27) | 1,641 (3.67) | |
| Unknown | 37,068 (74.55) | 3,781 (75.89) | 33,287 (74.4) | |
| Hist.type, n (%) | 0.006 | |||
| Others | 20,248 (40.72) | 2,120 (42.55) | 18,128 (40.52) | |
| PTC_NOS | 29,475 (59.28) | 2,862 (57.45) | 26,613 (59.48) | |
| Surgery.method, n (%) | <0.001 | |||
| None | 911 (1.83) | 211 (4.24) | 700 (1.56) | |
| Others | 8,105 (16.3) | 985 (19.77) | 7,120 (15.91) | |
| Subtotal thyroidectomy | 1,096 (2.2) | 116 (2.33) | 980 (2.19) | |
| Total thyroidectomy | 39,611 (79.66) | 3,670 (73.67) | 35,941 (80.33) | |
| Radiotherapy, n (%) | <0.001 | |||
| No/unknown | 31,225 (62.8) | 3,647 (73.2) | 27,578 (61.64) | |
| Yes | 18,498 (37.2) | 1,335 (26.8) | 17,163 (38.36) | |
| Chemotherapy, n (%) | <0.001 | |||
| No | 49,645 (99.84) | 4,960 (99.56) | 44,685 (99.87) | |
| Yes | 78 (0.16) | 22 (0.44) | 56 (0.13) | |
| Survival.months, median [Q1, Q3] | 42 [25, 61] | 41 [24, 59] | 43 [25, 61] | <0.001 |
| Outcome, n (%) | <0.001 | |||
| 0 | 48,624 (97.79) | 4,508 (90.49) | 44,116 (98.6) | |
| 1 | 1,099 (2.21) | 474 (9.51) | 625 (1.4) | |
| Outcome2, n (%) | <0.001 | |||
| 0 | 49,682 (99.92) | 4,964 (99.64) | 44,718 (99.95) | |
| 1 | 41 (0.08) | 18 (0.36) | 23 (0.05) | |
| Outcome3, n (%) | <0.001 | |||
| 0 | 48,624 (97.79) | 4,508 (90.49) | 44,116 (98.6) | |
| 1 | 41 (0.08) | 18 (0.36) | 23 (0.05) | |
| 2 | 1,058 (2.13) | 456 (9.15) | 602 (1.35) | |
Outcome: overall survival (0, alive; 1, deceased); Outcome2: disease-specific survival (0, others; 1, died of DTC); Outcome3: competing risk outcome (0, survived; 1, died of DTC; 2, died of other causes). AJCC, American Joint Committee on Cancer; Hist.type, histological type; Is.primary, prior malignancy history (Yes, without prior malignancy history; No, with prior malignancy history); NOS, nothing otherwise special; Q1, 25th percentile; Q3, 75th percentile; PTC, papillary thyroid cancer.
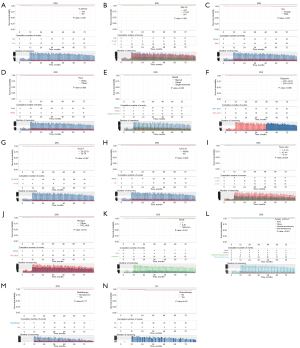
Identification of prognostic factors of OS and DSS in patients with stage I DTC in Kaplan-Meier analyses
Kaplan-Meier analyses were utilized to evaluate prognostic factors, including “Is.primary”, “Age.cat”, “Sex”, “Race”, “Marital”, “Diagnosis”, “AJCC.T”, “AJCC.N”, “Tumor.size”, “Hist.type”, “Grade”, “Surgery.method”, “Radiotherapy”, and “Chemotherapy” in enrolled patients. OS and DSS were compared. The cumulative number of events and the censoring number were also calculated. The Kaplan-Meier curves for OS and DSS of the factors are shown in Figure 1 and Figure 2. Several factors impacted OS, including “Is.primary” (P<0.001, Figure 1A), “Age.cat” (P<0.001, Figure 1B), “Sex” (P<0.001, Figure 1C), “Marital” (P<0.001, Figure 1E), “AJCC.T” (P<0.001, Figure 1G), “AJCC.N” (P<0.001, Figure 1H), “Tumor.size” (P<0.001, Figure 1I), “Surgery.method” (P<0.001, Figure 1L), “Radiotherapy” (P<0.001, Figure 1M) and “Chemotherapy” (P<0.001, Figure 1N). Factors impacted DSS including “Is.primary” (P<0.001, Figure 2A), “Age.cat” (P<0.001, Figure 2B), “Sex” (P<0.001, Figure 2C), “Marital” (P=0.006, Figure 2E), “AJCC.T” (P=0.007, Figure 2G), “AJCC.N” (P=0.005, Figure 2H), “Tumor.size” (P=0.024, Figure 2I), “Hist.type” (P=0.013, Figure 2J), “Grade” (P<0.001, Figure 2K), “Surgery.method” (P=0.001, Figure 2L) and “Chemotherapy” (P<0.001, Figure 2N).

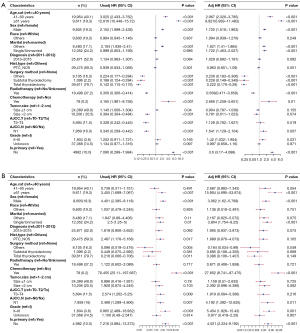
Identification of prognostic factors of OS and DSS in patients with stage I DTC in the Cox regression analyses
The clinicopathological features mentioned in Figure 1 and Figure 2 were considered possible predictors. To identify parameters influencing OS and DSS, univariate and multivariate Cox proportional hazards regression analyses were performed. Forest plots were used to display the results, and the corresponding information is detailed in Figure 3 (Figure 3A: OS; Figure 3B: DSS). The variable “Is.primary” was associated with the outcomes OS and DSS in univariate and multivariate Cox proportional hazards regression analyses. Multivariate regression analysis revealed that, after controlling for the effect of the other factor, the status of “Is.primary: No” was an independent factor for poor OS [hazard ratio (HR) =3.6, 95% confidence interval (CI): 3.17−4.088, P<0.001] and poor DSS (HR =4.521, 95% CI: 2.224−9.192, P<0.001).
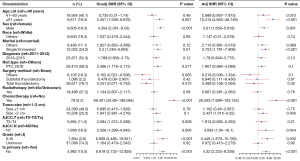
Identification of prognostic factors in patients with stage I DTC in the competing risk model
In the competing risk model, in both the univariate analysis and the multivariate analysis, we found that the patients in the “Is.primary: No” group had a higher risk of death from DTC than the patients in the “Is.primary: Yes” group [multivariate analysis: subdistribution HR (SHR) =4.32, 95% CI: 2.233−8.359, P<0.001, Figure 4]. Moreover, in addition, we found that age 61 years and above, male sex, being single or unmarried, receiving chemotherapy, tumor size more than 2 cm, AJCC N1, and grade II–III were risk factors for patient survival in the multivariate analysis of the competing risk model (all P<0.05, Figure 4).
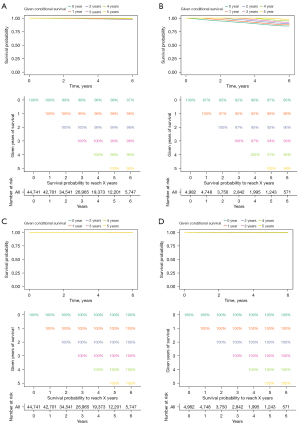
Conditional survival analysis in patients with stage I DTC
Conditional survival analysis was performed with the patients divided into 2 groups by the variable “Is.primary”. Conditional OS and DSS probabilities are shown in Figure 5. For the patients “Is.primary: Yes” (without a prior malignancy history), the probability of achieving 5-year survival increased from 98% to 98%, 99%, 99% and 99% given that 1, 2, 3 and 4 years were already survived respectively. Subjects who already survived 2 years had an additional 3‐year survival probability of 99%. It is higher than the initial and unconditional 5‐year survival estimate of 98%. However, the improvement of conditional OS only appeared with 2 years that had already survived. The conditional OS did not continue to improve with 3 and 4 years (Figure 5A). For the patients “Is.primary: No”, the trends that conditional OS was improved with prolonged survival time is more obvious than that for the patients “Is.primary: Yes”. The probability of achieving 5-year survival increased from 87% to 89%, 92%, 94% and 97% given that 1, 2, 3 and 4 years had already been survived, respectively. The more years patients had already survived, the better their chances of additional years of OS (Figure 5B). The conditional DSS of the two groups, “Is.primary: Yes” (Figure 5C) and “Is.primary: No” (Figure 5D), did not change with prolonged survival time because the initial and unconditional 5‐year survival estimates of the two groups were 100%.
Discussion
With the increasing incidence of tumors and development of screening, more patients suffer from multiple cancers (17-19). For the follow-up and prognosis of these patients with multiple cancers, however, only limited observations have been reported (20). Mortality from thyroid cancer was only 0.5 deaths per 100,000 (21). For DTC, it has a better prognosis, especially early-stage DTC. Both patients <55 years and ≥55 years have an estimated 10-year disease-specific survival of 98% to 100% for stage I DTC (4,22). Except for pathologic staging or clinical staging, factors that influence DTC prognosis are age, sex (23), surgical approach (24), tumor size and lesion number (25). Ito et al. found that a tumor size larger than 2 cm had an independent prognostic impact on recurrence and multivariate analysis showed that tumor multiplicity was a moderate prognostic factor (25). Subsequent studies in patients with low-risk papillary thyroid microcarcinoma (PTMC) without surgery showed that a younger age (<40 years) is an independent predictor of disease progression, whereas older patients (≥60 years) with subclinical low-risk PTMC may be the best candidates for observation rather than surgery (14).
Primary multiple tumor (PMT) is defined as an independent appearance and development of two or more tumors that arise simultaneously or at regular intervals in one patient (26). Patients with prior malignancy history have a 10% higher risk of developing a second tumor with respect to the general population (17,18). The estimated 10-year cumulative risk of second primary cancers for patients who were first diagnosed with cancer within age 60 to 69 was as high as 13% (19).
The incidence of PMT and predilection sites have been reported to vary in different studies. A study from China showed that PMT were observed more frequently in patients with head and neck tumors (5.65%) and urinary tumors (4.19%). Females were slightly more likely to suffer from PMT than males, with a ratio of 1.2 (27). Another study based on Chinese showed that the most common primary cancers were of digestive, breast, and lung origins for metachronous PMT, while the secondary ones were of digestive, lung, and urinary origins (28). Wang et al. showed that in female patients with a prior lung cancer history, thyroid cancer was the second most common concomitant tumor (20). In metachronous carcinoma PMT, breast cancer and gastric cancer are the most common primary neoplasms, while lung cancer and esophageal cancer are the most common secondary neoplasms (29).
In our study, we used population-based SEER data to build the competing risk model. In this model, we found that age 61 years and above, male sex, being single or unmarried, receiving chemotherapy, tumor size more than 2 cm, and grade II–III were risk factors for patient survival. These conclusions are consistent with existing reports. Importantly, we found that patients with a prior malignancy history had a higher risk of death from DTC than those without prior malignancy history. Synchronous PMT has previously been reported to be an independent factor for the inferior prognosis of lung cancer patients (20). However, to date, no evidence for the prognostic value of a prior malignancy history in stage I DTC has been reported in the literature.
In our study, we also focused on the dynamic changes in the prognosis of DTC. Conditional survival analysis was used for the dynamic evaluation of prognosis. For DTC patients, the risk of recurrence and disease-specific mortality change with longer follow-up, but no available and effective methods have been established to estimate the risk continually modified during follow-up (5). In our study, we found that for the stage I DTC patients, because of low DTC-related death (3,4), the conditional DSS was not improved for either the patients with a prior malignancy history or those without. Both patients with or without prior malignancies has improved conditional OS. The patients without prior malignancy history only had an improvement at the 2-year given conditional OS. For the patients with a prior malignancy history, the conditional OS improvement was more pronounced.
However, our research has some limitations. First, the staging system that we used was the 7th edition of the AJCC staging system. Additionally, our study is a retrospective study, and due to the limitations of the SEER database, some prognostic factors were not included in our study. Suppression of thyroid-stimulating hormone (TSH) is one of the mainstays of DTC treatment (3). As we cannot obtain information about the suppression of TSH from SEER, the relationship between the prior malignancy history and prognosis cannot be fully elucidated by SEER analyses.
Conclusions
In summary, our study showed that a prior malignancy history was linked to an adverse survival of stage I DTC patients, and for patients with prior malignancy history, the longer the follow-up, the better the OS was. Conditional survival can directly represent dynamic changes in prognosis of individuals who have survived a certain period after being diagnosed with stage I DTC, and thus it is useful for the development of thyroid cancer long-term follow-up strategies.
Acknowledgments
The authors thank the creators and maintainers of SEER database.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2232/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-2232/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chou R, Dana T, Haymart M, et al. Active Surveillance Versus Thyroid Surgery for Differentiated Thyroid Cancer: A Systematic Review. Thyroid 2022;32:351-67. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Schlumberger M, Leboulleux S. Current practice in patients with differentiated thyroid cancer. Nat Rev Endocrinol 2021;17:176-88. [Crossref] [PubMed]
- Tam S, Boonsripitayanon M, Amit M, et al. Survival in Differentiated Thyroid Cancer: Comparing the AJCC Cancer Staging Seventh and Eighth Editions. Thyroid 2018;28:1301-10.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Pashayan N, Morris S, Gilbert FJ, et al. Cost-effectiveness and Benefit-to-Harm Ratio of Risk-Stratified Screening for Breast Cancer: A Life-Table Model. JAMA Oncol 2018;4:1504-10. [Crossref] [PubMed]
- Xia JY, Aadam AA. Advances in screening and detection of gastric cancer. J Surg Oncol 2022;125:1104-9. [Crossref] [PubMed]
- Fitzgerald RC, Antoniou AC, Fruk L, et al. The future of early cancer detection. Nat Med 2022;28:666-77. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Rosso S, De Angelis R, Ciccolallo L, et al. Multiple tumours in survival estimates. Eur J Cancer 2009;45:1080-94. [Crossref] [PubMed]
- Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res 2010;174:741-52. [Crossref] [PubMed]
- Lal G, Groff M, Howe JR, et al. Risk of subsequent primary thyroid cancer after another malignancy: latency trends in a population-based study. Ann Surg Oncol 2012;19:1887-96. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014;24:27-34. [Crossref] [PubMed]
- Glikson E, Alon E, Bedrin L, et al. Prognostic Factors in Differentiated Thyroid Cancer Revisited. Isr Med Assoc J 2017;19:114-8. [PubMed]
- Orosco RK, Hussain T, Brumund KT, et al. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid 2015;25:125-32. [Crossref] [PubMed]
- Xu LL, Gu KS. Clinical retrospective analysis of cases with multiple primary malignant neoplasms. Genet Mol Res 2014;13:9271-84. [Crossref] [PubMed]
- Italian cancer figures, report 2013: Multiple tumours. Epidemiol Prev 2013;37:1-152. [PubMed]
- Tabuchi T, Ito Y, Ioka A, et al. Incidence of metachronous second primary cancers in Osaka, Japan: update of analyses using population-based cancer registry data. Cancer Sci 2012;103:1111-20. [Crossref] [PubMed]
- Wang H, Hou J, Zhang G, et al. Clinical characteristics and prognostic analysis of multiple primary malignant neoplasms in patients with lung cancer. Cancer Gene Ther 2019;26:419-26. [Crossref] [PubMed]
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164-7. [Crossref] [PubMed]
- Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017;27:751-6.
- Suteau V, Munier M, Briet C, et al. Sex Bias in Differentiated Thyroid Cancer. Int J Mol Sci 2021;22:12992. [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. Erratum in: Thyroid 2010 Aug;20(8):942. Hauger, Bryan R [corrected to Haugen, Bryan R]; Thyroid. 2010 Jun;20(6):674-5. [Crossref] [PubMed]
- Ito Y, Kudo T, Kihara M, et al. Prognosis of low-risk papillary thyroid carcinoma patients: its relationship with the size of primary tumors. Endocr J 2012;59:119-25. [Crossref] [PubMed]
- Romaniuk A, Lyndin M, Smiyanov V, et al. Primary multiple tumor with affection of the thyroid gland, uterus, urinary bladder, mammary gland and other organs. Pathol Res Pract 2017;213:574-9. [Crossref] [PubMed]
- Liu Z, Liu C, Guo W, et al. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One 2015;10:e0125754. [Crossref] [PubMed]
- Wang Y, Jiao F, Yao J, et al. Clinical Features of Multiple Primary Malignant Tumors: A Retrospective Clinical Analysis of 213 Chinese Patients at Two Centers. Discov Med 2021;32:65-78. [PubMed]
- Si L, Feng Y, Wang Y, et al. Clinical and pathological characteristics of multiple primary malignant neoplasms cases. Int J Clin Pract 2021;75:e14663. [Crossref] [PubMed]


