The role of nuclear factor-kappa B and endoplasmic reticulum stress in hepatitis B viral-induced hepatocellular carcinoma
Hepatocellular carcinoma (HCC) ranks fifth among the most common solid tumours and second most common cause of cancers-related death (1,2). The number of new HCC cases diagnosed each year is estimated to be half a million worldwide. Due to the high mortality rate of HCC, the occurrence rate is almost equivalent to the mortality rate and there are very limited treatment available (2).
Although the pathogenesis of HCC has been studied in depth for decades, the exact mechanisms and pathways resulting in its development are still unclear and warrant further investigation. Since 1970s, chronic infection with hepatitis B virus (HBV) was reported to be associated with the development of HCC (2). In fact, HBV is the single most common cause of HCC that accounts for approximately 50% of all HCC cases worldwide, especially in developing countries (3,4). There are 350 million individuals worldwide infected with HBV that are at greater risk of HCC (1). HBV-related HCC has extremely poor prognosis with median survival of less than 16 months (1). Population-wide vaccination programs against HBV have been linked to significant reductions in incidence of HCC (2). Long-term nucleos(t)ide analogue (NA) therapy that controls HBV replication and suppresses HBV viremia also displayed high efficacy in reducing risk of HCC (5). Despite that, the occurrence of HCC in inactive chronic hepatitis B (albeit in the absence of cirrhosis and inflammation) is still observed in 10–20% of the patients with chronic HBV infection, especially those with high HBsAg levels (6).
The nuclear factor-kappa B (NF-κB) signalling pathway plays a central function in liver homeostasis, pathophysiology and regulation of the inflammation-cancer axis. The NF-κB transcription factor family in mammals consists of five proteins, p65 (RelA), RelB, c-Rel, p105/p50 (NF-κB1), and p100/52 (NF-κB2), which can form large numbers of homo- and hetero-dimers (7). In unstimulated cells, the NF-κB dimers are sequestered in the cytoplasm by inhibitor of KB (IKBs) (8). IKBs are degraded by upstream kinase complex IκB kinases (IKKs) upon activation thereby allowing nuclear translocation of NF-κB dimers and the subsequent induction of NF-κB response genes. IKK1/IKKα, IKK2/IKKβ, and NEMO/IKKγ appear to be the master regulators of NF-κB activation and their downstream effects including cellular proliferation, apoptosis, and inflammation, all of which play central roles in hepatocarcinogenesis (7). There are two NF-κB pathways: (I) the NF-κB canonical pathway which relies on the inducible degradation of inhibitory IκB proteins, particularly IκBα which retains most NF-κB dimers in the cytoplasm; (II) the alternative pathway which depends on the inducible processing of NF-κB2 precusor protein p100 to its mature p52 in an IKK1/IKKα depedent manner (9).
The link between hepatitis B protein and other viral proteins with NF-κB function during the course of chronic B hepatitis was discussed in many previous studies (10). Notably, the hallmark of first-line antiviral host defence response entails the release of NF-κB-dependent interferons and cytokines, including tumour necrosis factor alpha (TNFα), IL-1β and IL-1α. NF-κB activation is often observed in hepatitis associated HCC as well (4,7,11,12). NF-κB induces the expression of a number of genes which can directly or indirectly suppress apoptosis, including inhibitors of apoptosis, c-FLIP, TRAF1, TRAF2, IEX-1, and ferritin heavy chain (13). The ablation of NF-κB regulators in mouse models leads to spontaneous liver injury, fibrosis and HCC. Many molecules and pathways that are linked to HCC are either targets or activators of NF-κB. For instance, Pikarsky et al. demonstrated that NF-κB is essential for promoting inflammation-associated cancer in a TNFα driven manner (10,12). Also, Inokuchi et al. showed that TGF-β-activated kinase-1 (TAK1), an activator of NF-κB, promotes spontaneous hepatocyte death which subsequently leads to inflammation, fibrosis, and ultimately to hepatocarcinogenesis via a TNF receptor dependent mechanism (14). On the other hand, HBx proteins can directly trigger carcinogenesis by blocking of TNFα and FAS-mediated apoptosis through a NF-κB dependent manner (11). Indeed, there are strong evidences of direct effect of HBV itself, even without the related cirrhosis or inflammation, in causing HCC (3,4).
Apart from NF-κB activation, endoplasmic reticulum (ER) stress is also linked to liver carcinogenesis (15,16). The presence of ground glass hepatocytes is a typical feature of chronic HBV infection which consists of accumulated mutated surface antigen within the ER lumen. The accumulation of these unfolded or misfolded proteins in the ER triggers the unfolded protein response (UPR) which aims to restore the normal protein function by either degrading them, activating the molecular chaperones involved in proper protein folding or initiates apoptosis when the ER stress is too severe (15). However pro-longed UPR is oncogenic where there is accumulation of DNA damage and dysregulation of cell proliferation and survival (16). ER stress and UPR associated genes such as activating transcription factor (ATF), X-box binding protein-1 (XBP1) and CHOP (GADD153/DDIT3, growth arrest and DNA damage-inducible gene 153) are found to be associated with liver carcinogenesis (17). The link between NF-κB activation, ER stress, and liver damage is well established (10,18). For instance, CHOP induces cell death and inflammatory responses via activation of NF-κB through a pathway involving IRAK2-induced secretion of proinflammatory cytokines, IL-8 and TNFα (18). On the other hand, NF-κB can also be activated by the Inositol requiring 1α (IRE1α), an ER stress sensor (13). IRE1α initiates the cleavage of 26 nucleotides from X-box binding protein-1 (XBP1) mRNA in the cytosol. After splicing, XBP1 mRNA encodes a potent transcription factor (sXBP1) which activates a subset of UPR genes that are involved in ER biogenesis and ER-associated degradation (ERAD).
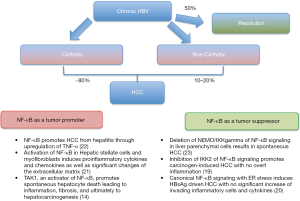
Interestingly, cases of patients with chronic hepatitis B and immune suppression suggest that in the absence of adaptive immune responses, cellular stress induced by HBV sufficiently drives liver disease and carcinogenesis (19-22). As shown in Figure 1, HBV-related HCC may occur in the absence of cirrhosis, even though the majority (up to 80%) of the cases of HBV-related HCC occur in association with cirrhosis (3,4). As mentioned above, the liver inflammation, injury, cirrhosis and cancer are deemed to be linked to the activation of NF-κB signalling in many studies (4,12,14). There are studies, however pointed out the opposite direction. For instance, liver-specific inhibition of NF-κB signalling by conditional ablation of IKK2 promotes carcinogen-induced liver cancer (19). Moreover, inhibition of NF-κB by deletion of NEMO (the regulatory IKK subunit) in parenchymal liver cells led to spontaneous hepatocarcinogenesis in mice (4). Possibly, NF-κB inhibition in some hepatocytes may cause apoptosis and regenerative responses which leads to survival and malignant transformation. It is also possible that NF-κB functions are not homogenous within one cell population within different cellular compartments. Alternatively, NF-κB plays a distinct role in different stages of the hepatocarcinogenesis (4). This suggests that the role of NF-κB signalling in HBV associated HCC is complex and could be regarded as a double-edged sword which would be better dissected in a spatial-temporal manner. Their multiple hepatocarcinogenesis mechanisms have been elucidated via a few important studies (4,12,14,17,19,23) including the one by Sunami et al. in Hepatology 2015 (20) (Figure 1).
The study by Sunami et al. (20) aimed to address the question of whether NF-κB signaling could play an oncogenic or suppressive role in hepatocytes expressing high level of viral transcripts in the absence of specific inflammation. First, they observed nuclear translocation of RelA/p65 in patients who were chronically infected with HBV, including in inactive carriers (hepatitis B envelop antigen-negative, showing no significant necroinflammation, and HBV DNA <2,000 IU/mL). Then the authors adopted a well-characterized transgenic mouse model which exhibits hepatocyte-specific expression of L, M, and S HBV surface proteins at high levels in the absence of specific inflammation. Liver cells injuries were observed by 4 months and HCC were developed (with an incidence of approximately 50%) by 12–20 months due to the accumulation of HBV surface proteins (HBsAg). RelA/p65-positive hepatocyte nuclei and the activation of canonical NF-κB signalling were also detected. These data suggested that without active inflammation, overexpression of HBsAgs alone could sufficiently trigger canonical NF-κB signalling activation and tumor development in hepatocytes. This is in consistent with the previous studies by the authors demonstrating canonical NF-κB signalling activation in hepatocytes of patients with inactive chronic HBV infection (19). In order to further examine the role of NF-κB signaling, they generated a model by crossing HBsAg+ transgenic mice to mice that expressed a dominant negative form of IKK2/IKKβ (IKK2KD) in hepatocytes (IKK2KDHep mice), which results in the hepatocyte-specific inhibition of canonical NF-κB signalling pathway. HBsAg+/IKK2KDHep mice showed a dramatic increase in the number and size of tumour as opposed to HBsAg+ single-transgenic animals which displayed small tumour nodules. A drastic increase of tumour incidence to 100% was also observed at 70 weeks as compared to 50% in HBsAg single-transgenic mice. On the other hand, 70-week-old IKK2KDHep single-transgenic mice showed small adenomas in one of six animals, supporting the crucial role of canonical NF-κB signalling in liver tumourgenesis.
Intriguingly, all these results occurred in the absence of significant increase in both the invading inflammatory cells such as CD3+ T cells and F4/80+ macrophages, and the expression of the inflammatory cytokine IL-6. This suggests that the HBsAg and canonical NF-κB signalling could enhance tumorgenesis without the presence of adaptive or active inflammation. This was later shown to be related to the induction of ER stress as evidenced by the uneven distribution of HBsAg throughout livers as a result of defects in protein processing. The authors then further investigated the expression of several genes known to be involved in ER stress and UPR. Among the downregulated genes was BiP/GRP78, a molecular chaperone which is involved in ER stress-associated protein degradation and the subsequent cell survival or apoptosis (15). In many previous studies, BiP has been shown to be the central regulator in ER stress sensing and UPR, and having a role in liver carcinogenesis (16). Reduction in BiP leads to deregulation of a number of other ER-related proteins or genes. As reported by Sunami et al., these include an increased processed ATF6, enhanced eIF2 phosphorylation, elevated ATF4 and CHOP leading to sustained ER stress response (20). Overexpression of CHOP has previously been shown to block cells from progressing from the G1 to the S phase (24). In this study, it was demonstrated that the sustained CHOP expression was associated with inadequate liver regeneration accompanied by overexpression of p27 (20). The ability of p27 to block cyclin D/CDK4 and CDK2 activity was shown to be linked to tumorgenesis (25). Moreover, ATF3, which supress cyclin D1 and CHOP expression was also decreased in the HBsAg+/IKK2KDHep compared to HBsAg+ single-transgenic mice.
All these results presented by Sunami et al. suggest the disruption of UPR and the loss of ER stress control which possibly contribute to G1/S phase cell cycle arrest, severe cellular stress, inadequate liver regeneration, uncontrolled ER stress and eventually the development of HCC. Last but not least, the authors also suggested that the absence of canonical NF-κB signalling disrupts cellular stress responses that would be triggered by ER stress and increase the sporadic occurrence of DNA damage which must be tightly regulated to avoid loss of function and tumorigenesis.
In summary, the study by Sunami et al. provided important evidence on the critical role of canonical NF-κB signalling in HBsAg-driven HCC by controlling the UPR. The lack of canonical NF-κB signalling caused a sustained and uncontrolled UPR with loss of BiP, overexpression of CHOP, cell cycle arrest and the consequential cellular stress. This is demonstrated with the observation of accumulation of DNA damage and 100% HCC incidence in the current HBsAg+/IKK2KDHep model. However, before translating this study into any clinical application, further studies are necessary to determine whether these mechanisms also exist in human and if they can be extended to other hepatitis infection such as HCV.
AcknowledgmentsOther Section
Funding: This article is supported by National Medical Research Council (NMRC), Singapore (reference numbers: MOHIAFCAT2001, NMRC/STaR/020/2013) and Biomedical Research Council (BMRC) (reference number: EDB IAF 311020).
FootnoteOther Section
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chunying Liu (Department of Molecular Oncology, Eastern Hepatobiliary Surgical Hospital National Center of Liver Cancer, Second Military Medical University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.06.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat 2009;16:453-63. [Crossref] [PubMed]
- Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology 2009;49:S56-60. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011;8:108-18. [Crossref] [PubMed]
- Cho JY, Paik YH, Sohn W, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut 2014;63:1943-50. [Crossref] [PubMed]
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49:S45-55. [Crossref] [PubMed]
- Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene 2008;27:6228-44. [Crossref] [PubMed]
- Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NF-kappaB complex. Cell 1998;95:749-58. [Crossref] [PubMed]
- Sun SC. Non-canonical NF-κB signaling pathway. Cell Res 2011;21:71-85. [Crossref] [PubMed]
- Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J 1995;14:2580-8. [PubMed]
- Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol 2016;64:S84-S101. [Crossref] [PubMed]
- Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461-6. [Crossref] [PubMed]
- Hu P, Han Z, Couvillon AD, et al. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol 2006;26:3071-84. [Crossref] [PubMed]
- Inokuchi S, Aoyama T, Miura K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A 2010;107:844-9. [Crossref] [PubMed]
- Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer 2014;14:581-97. [Crossref] [PubMed]
- Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 2011;54:795-809. [Crossref] [PubMed]
- Updegraff BL, O'Donnell KA. Stressing the importance of CHOP in liver cancer. PLoS Genet 2013;9:e1004045 [Crossref] [PubMed]
- Willy JA, Young SK, Stevens JL, et al. CHOP links endoplasmic reticulum stress to NF-κB activation in the pathogenesis of nonalcoholic steatohepatitis. Mol Biol Cell 2015;26:2190-204. [Crossref] [PubMed]
- Maeda S, Kamata H, Luo JL, et al. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005;121:977-90. [Crossref] [PubMed]
- Sunami Y, Ringelhan M, Kokai E, et al. Canonical NF-κB signaling in hepatocytes acts as a tumor-suppressor in hepatitis B virus surface antigen-driven hepatocellular carcinoma by controlling the unfolded protein response. Hepatology 2016;63:1592-607. [Crossref] [PubMed]
- Mason AL, Wick M, White HM, et al. Increased hepatocyte expression of hepatitis B virus transcription in patients with features of fibrosing cholestatic hepatitis. Gastroenterology 1993;105:237-44. [Crossref] [PubMed]
- Meuleman P, Libbrecht L, Wieland S, et al. Immune suppression uncovers endogenous cytopathic effects of the hepatitis B virus. J Virol 2006;80:2797-807. [Crossref] [PubMed]
- Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007;11:119-32. [Crossref] [PubMed]
- Barone MV, Crozat A, Tabaee A, et al. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev 1994;8:453-64. [Crossref] [PubMed]
- Kato JY, Matsuoka M, Polyak K, et al. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 1994;79:487-96. [Crossref] [PubMed]