
A new risk algorithm combining D-dimer and HE4 differentiates borderline tumor from patients with ovarian tumor
Highlight box
Key findings
• D-dimer (DD) is an important serological marker that can be used for the diagnosis of borderline ovarian tumors (BOTs). When used in combination with human epididymis protein 4 (HE4), it has higher diagnostic value.
What is known and what is new?
• DD has been linked to several disorders, including thrombotic diseases and coagulation dysfunction. However, research on the role of BOTs is limited.
• DD is a potential diagnostic marker for BOTs. Combining it with HE4 in real-time further enhances the diagnostic accuracy.
What is the implication, and what should change now?
• Using DD can increase the diagnostic efficiency of BOTs.
Introduction
Borderline ovarian tumors (BOTs) were first proposed by Taylor in 1929 and redefined by the World Health Organization (WHO) in 2014 as tumors with atypical proliferative characteristics but lacking invasive mesenchymal infiltration (1,2). These tumors exhibit cytologic features suggestive of malignancy and have a slow clinical progression (3). Detecting BOTs is challenging due to their unique pathological characteristics, especially when relying on non-invasive methods such as diagnostic markers and imaging (4-6). As the tumor volume increases, it negatively impacts ovarian function, highlighting the importance of early diagnosis and treatment to preserve ovarian function (7,8). Therefore, an accurate diagnosis of BOTs is critical for maintaining ovarian function and improving prognosis.
Unfortunately, research on the diagnosis of BOTs is lacking. Commonly used diagnostic markers and risk prediction models are borrowed from malignant ovarian tumors, such as carbohydrate antigen 125 (CA125), human epididymis protein 4 (HE4), and risk of ovarian malignancy algorithm (ROMA) (9-11). Recent study suggests that their clinical utility is limited. While imaging techniques like transvaginal ultrasound (TVUS) and magnetic resonance imaging (MRI) are employed for ovarian tumor diagnosis, their accuracy depends on the expertise of radiologists, restricting their accessibility (12). Despite the limited practical value of HE4 and CA125, exploring small-molecule markers for disease diagnosis is warranted due to their non-invasiveness, simplicity, and reliability (8).
The blood clotting system plays a crucial role in maintaining normal physiological function and can be altered with pathological processes like tumorigenesis (13). Tumor cells can trigger the clotting-fibrinolytic system, leading to the release of various procoagulant, fibrinolytic markers, and hemostatic factors, which can promote neoangiogenesis by stimulating vascular endothelial cell proliferation and differentiation (14-16). This stimulation further facilitates tumor growth, invasion, metastasis, and recurrence. D-dimer (DD) as a product of fibrinogen (FIB) breakdown, is a biomarker of abnormal coagulation function widely accepted for diagnosing and predicting the prognosis of several malignancies (17-19). Notably, DD levels have a direct positive correlation with CA125 or HE4 levels (20). As such, DD may play a role in managing BOTs. Additionally, FIB and platelet (PLT) count can reflect changes in coagulation function and serve as markers to predict cancer prognosis. Homocysteine (HCY) is closely associated with tumor progression in ovarian cancers, with its diagnostic value previously confirmed in research. Therefore, DD, FIB, PLT, and HCY are potential markers for diagnosing BOTs, and this study aims to verify their utility in diagnosing BOTs.
Given these considerations, CA125, HE4, DD, FIB, PLT, and HCY were selected as candidate markers for diagnosing BOTs. The goal of this study is to validate the effectiveness of these candidate diagnostic markers for BOTs and determine the optimal combination of markers to enhance detection rates. We present this article in accordance with the STARD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1276/rc).
Methods
Patients
From January 2016 to December 2019, this study included 83 patients with BOTs who had undergone surgery at Women’s Hospital of Nanjing Medical University (Nanjing Maternity and Child Health Care Hospital), with complete clinical data available for 55 of them who met the inclusion criteria. Additionally, 55 patients with benign ovarian tumors who visited our hospital during the same period were randomly selected as controls (Figure 1). All patients were diagnosed by two or three independent pathologists through pathological diagnosis. The clinical data of all included patients were retrospectively collected. Patients with incomplete information were excluded. The inclusion criteria for BOTs, while for benign tumor patients, it was benign tumor of the ovary. Exclusion criteria included malignant tumors (past or concurrent), severe comorbidities (such as poorly controlled hypertension or diabetes), liver and blood system diseases, history of thrombosis, history of medication affecting blood clotting in the last 6 months. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Women’s Hospital of Nanjing Medical University (No. 2022KY-018) and was registered at the Chinese Clinical Trial Registry (registration No. ChiCTR1900023149). Individual consent for this retrospective analysis was waived.
Candidate marker serum concentration
Blood samples were quickly collected prior to treatment, in order to test for CA125, HE4, DD, FIB, PLT, and HCY levels. The COBAS 6000 analyzer (Roche, Basel, Switzerland) and corresponding reagent kit were used to measure the levels of CA125 and HE4 in the serum. The levels of DD and FIB before surgery were measured using the CA7000 automated coagulation analyzer (Sysmex, Kobe, Japan) and specific reagents. Special equipment and matching kits were utilized to measure PLT and HCY levels.
Statistical analysis
We used SPSS 23 (Chicago, IL, USA), GraphPad Prism 7.0 software (version VII; La Jolla, CA, USA), and MedCalc Statistical Software version 15.6.1 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015) for data analysis. Normally distributed data were presented as mean and standard deviation (SD), while non-normally distributed data was presented as median and interquartile range (IQR). We assessed the differences between groups using the Mann-Whitney U test. Logistic regression analysis was used to evaluate the relationship between markers and BOTs and to construct a new risk forecast model. We assessed the calibration of the model using the Hosmer Lemeshow χ2 test. We assessed the diagnostic efficacy of the candidate markers using the receiver operating characteristic (ROC) curve, Youden index, the area under the ROC curve (AUC), sensitivity, and specificity. Sensitivity and specificity were estimated based on the ideal cutoff. P value <0.05 was considered statistically significant. If requested, we can provide the data in this study to test its reproducibility.
Results
A total of 83 patients diagnosed with BOTs were included at the beginning of the experiment. After screening, 28 patients were excluded based on predetermined criteria. Ultimately, the study included 55 patients with BOTs and 55 randomly selected patients with benign ovarian tumor. The baseline characteristics of all patients were recorded and presented in Table 1 and Figure 2. Significantly higher level of CA125, HE4, FIB, and DD were observed in the BOTs compared to the control group. This led to further investigation into the diagnostic value of these markers. Notably, DD showed the highest AUC area, indicating its strong diagnostic value (Table 2). All included markers had AUC areas greater than 0.5, suggesting their potential use in diagnosing BOTs. But it is worth noting except FIB, the diagnostic value of those three markers is no significant difference.
Table 1
| Characteristics | Borderline | Benign | P |
|---|---|---|---|
| Number | 55 | 55 | – |
| Age (years) | 36.509 (12.02) | 36.055 (11.967) | 0.84 |
| BMI (kg/m2) | 23.11 (4.211) | 22.5 (3.432) | 0.41 |
| CA125 (U/mL) | 40.46 [17.56–167.4] | 16.69 [13.07–26.01] | <0.001 |
| HE4 (pmol/L) | 56.8 [49.64–68.83] | 47.16 [41.34–58.31] | <0.001 |
| DD (mg/L) | 0.5 [0.25–0.76] | 0.19 [0.13–0.26] | <0.001 |
| FIB (g/L) | 2.511 [2.209–3.01] | 2.301 [2.065–2.6] | 0.01 |
| PLT (×109/L) | 247 [207–297] | 229 [203–268] | 0.17 |
| HCY (μmol/L) | 7.7 [6.37–9.8] | 6.8 [5.74–9.3] | 0.18 |
| ROMA | 10.94 [7.83–17.1] | 7.78 [5.41–11.11] | <0.001 |
| Menopausal status | 0.43 | ||
| Premenopausal | 48 | 45 | |
| Postmenopausal | 7 | 10 | |
| Maximum diameter of tumor (cm) | – | ||
| <4 | 19 | – | |
| ≥4 | 36 | – | |
Data are presented as number, mean (SD), or median [IQR]. BMI, body mass index; CA125, cancer antigen 125; HE4, human epididymis protein 4; DD, D-dimer; FIB, fibrinogen; PLT, platelet; HCY, homocysteine; ROMA, risk of ovarian malignancy algorithm; SD, standard deviation; IQR, interquartile range.
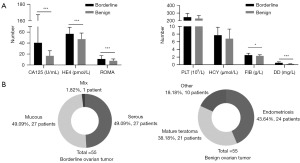
Table 2
| Characteristics | AUC | 95% CI | P† |
|---|---|---|---|
| DD | 0.818 | 0.733–0.885 | – |
| CA125 | 0.736 | 0.644–0.816 | 0.84 |
| HE4 | 0.725 | 0.631–0.806 | 0.10 |
| FIB | 0.636 | 0.539–0.726 | 0.005 |
†, other markers compared with DD. ROC, receiver operating characteristic curve; AUC, area under the ROC curve; CI, confidence interval; DD, D-dimer; CA125, cancer antigen 125; HE4, human epididymis protein 4; FIB, fibrinogen.
DD, CA125, and HE4 were significantly associated with BOTs in univariate analysis. And then, correlation has been confirmed in multivariate analysis, except CA125 (Table 3).
Table 3
| Marker | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Relative risk | 95% CI | P | Relative risk | 95% CI | P | ||
| DD | 1862.373 | 68.012–50,997.363 | 0.003 | 698.053 | 19.945–24,431.377 | <0.001 | |
| CA125 | 1.023 | 1.004–1.042 | 0.02 | – | |||
| HE4 | 1.074 | 1.033–1.116 | <0.001 | 1.044 | 1.003–1.086 | 0.04 | |
CI, confidence interval; DD, D-dimer; CA125, cancer antigen 125; HE4, human epididymis protein 4.
As a result, a new diagnostic model combining HE4 and DD was established, which showed good fit with the data according to the Hosmer-Lemeshow χ2 test (8 degrees of freedom, χ2=3.929, P=0.86).
The diagnostic accuracy of the risk algorithm was assessed using ROC, Youden index, sensitivity, and specificity. The results showed a significant improvement in diagnostic accuracy (Figure 3).

Additionally, ROMA and pelvic MRI was also examined. Excitingly, the risk algorithm was found to be more suitable for diagnosing BOTs (Figure 3) which was superior to ROMA and MRI.
Furthermore, subgroup analyses were conducted based on pathological type and tumor diameter. The risk algorithm performed better in diagnosing serous tumors and tumor diameter ≥4 cm (Figure 4, Table 4). However, when used for diagnosing patients with small tumor diameter (<4 cm), the AUC area was also greater than 0.75, meaning significant clinical value (Figure 4, Table 4).
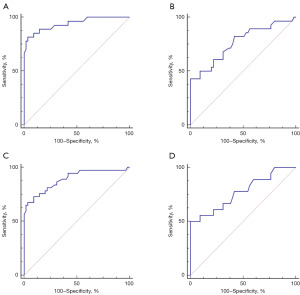
Table 4
| Characteristics | AUC (95% CI) | Youden index | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Pathologic type | ||||
| Serous tumors | 0.941 (0.866–0.981) | 0.786 | 81.48 | 96.36 |
| Mucous tumors | 0.765 (0.658–0.851) | 0.444 | 44.44 | 100 |
| Maximum diameter of tumor (cm) | ||||
| <4 | 0.772 (0.659–0.862) | 0.5 | 50 | 100 |
| ≥4 | 0.890 (0.808–0.946) | 0.640 | 67.57 | 98.36 |
AUC, area under the ROC curve; ROC, receiver operating characteristic; DD, D-dimer; HE4, human epididymis protein 4; BOT, borderline ovarian tumor; CI, confidence interval.
Discussion
BOTs represent 15% of ovarian epithelial tumors and are often asymptomatic in the early stages due to their complex anatomical location, but they have a favorable 5-year survival rate of almost 99% at stage I (21,22). Despite this, attention is warranted for two main reasons. Firstly, BOTs primarily affect women of reproductive age, threatening female fertility, essential for addressing declining fertility rates (23). Secondly, BOTs can potentially evolve into malignant tumors, especially when bilateral (24). Our study observed that the average age of patients with BOTs was 36.5 years, aligning with the childbearing age range (25). Therefore, determining the appropriate timing for surgery is crucial to protect fertility and prevent malignant progression. Identifying whether a tumor is physiological or pathological, particularly when small in size (<4 cm), can be challenging. Clinical monitoring is crucial as tumor growth can impact ovarian function and increase the risk of malignancy. Improving diagnostic efficiency, especially for small tumors, is vital.
About 30% of patients with BOTs are asymptomatic before diagnosis, while 50–60% experience non-specific symptoms like abdominal pain, distention, vaginal bleeding, and sexual discomfort (26). In a study of 151 women with BOTs, 84% reported these non-specific symptoms for an average of 6 months prior to diagnosis (27). Methods such as TVUS and MRI play crucial roles in diagnosing BOTs. A review found that MRI had 85% sensitivity and 74% specificity (28). Ultrasound can assist in the preoperative diagnosis of BOTs, although recent report suggests an accuracy of only 69% (29). Differentiating BOTs from benign or malignant ovarian tumors is challenging due to the lack of distinct morphological features. The ability of TVUS and MRI features to distinguish BOTs from benign or malignant ovarian tumors is limited by factors such as solid component size and septations’ thickness (30).
Research on BOTs diagnosis is lacking, representing a valuable area for exploration. Our study discovered that CA125 and HE4 levels were significantly higher in BOTs patients compared to those with benign tumors, in line with previous research (21,31,32). Additionally, differences in DD and FIB levels, often overlooked, were noted. DD displayed the best diagnostic efficiency according to ROC curve assessment (AUC: 0.818). Combining markers improved diagnostic accuracy, with a new formula based on DD and HE4 demonstrating excellent performance, surpassing ROMA and MRI (33). Subgroup analysis by pathological type or tumor diameter showed superior diagnostic performance, particularly for serous BOTs patients (AUC: 0.941) and those with smaller tumor diameters (<4 cm, AUC: 0.890). Notably, the new formula remained effective for small tumor diameter BOTs patients with an AUC of 0.772.
While our findings enhance the preoperative detection of BOTs, further improvements are needed to optimize diagnostic accuracy and prognosis. There are limitations in this study, including its retrospective nature, lack of replication in independent populations, and a small sample size. To validate the study’s accuracy, a large-scale multicenter prospective study is necessary.
Conclusions
In conclusion, in the absence of effective diagnostic markers, our research can proceed within certain parameters to improve the diagnostic efficiency of BOTs while validating our study’s conclusions.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1276/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1276/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1276/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1276/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Women’s Hospital of Nanjing Medical University (No. 2022KY-018) and was registered at the Chinese Clinical Trial Registry (registration No. ChiCTR1900023149). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lu Z, Chen J. Introduction of WHO classification of tumours of female reproductive organs, fourth edition. Zhonghua Bing Li Xue Za Zhi 2014;43:649-50.
- Taylor HC. Malignant and semimalignant tumors of the ovary. Surg Gynecol Obstet 1929;48:204-30.
- Sun Y, Xu J, Jia X. The Diagnosis, Treatment, Prognosis and Molecular Pathology of Borderline Ovarian Tumors: Current Status and Perspectives. Cancer Manag Res 2020;12:3651-9. [Crossref] [PubMed]
- Ye D, Shen H, Huang W, et al. A retrospective analysis of relapse-related factors for ovarian borderline tumors. Am J Transl Res 2022;14:5712-8. [PubMed]
- Tsuboyama T, Sato K, Ota T, et al. MRI of Borderline Epithelial Ovarian Tumors: Pathologic Correlation and Diagnostic Challenges. Radiographics 2022;42:2095-111. [Crossref] [PubMed]
- Eymerit-Morin C, Brun JL, Vabret O, et al. Borderline ovarian tumours: CNGOF Guidelines for clinical practice - Biopathology of ovarian borderline tumors. Gynecol Obstet Fertil Senol 2020;48:629-45. [PubMed]
- Haq SMA, Chowdhury MAF, Ahmed KJ, et al. Environmental quality and its impact on total fertility rate: an econometric analysis from a new perspective. BMC Public Health 2023;23:2397. [Crossref] [PubMed]
- Morotti M, Menada MV, Gillott DJ, et al. The preoperative diagnosis of borderline ovarian tumors: a review of current literature. Arch Gynecol Obstet 2012;285:1103-12. [Crossref] [PubMed]
- Qiao L, Chen X, Xi X, et al. Correlation analysis and clinical significance of CA125, HE4, DDI, and FDP in type II epithelial ovarian cancer. Medicine (Baltimore) 2020;99:e23329. [Crossref] [PubMed]
- Wu J, Zhang Y, Liu G, et al. New use of preoperative fibrinogen in ovarian cancer management. Transl Cancer Res 2023;12:3105-12. [Crossref] [PubMed]
- Luo HJ, Hu ZD, Cui M, et al. Diagnostic performance of CA125, HE4, ROMA, and CPH-I in identifying primary ovarian cancer. J Obstet Gynaecol Res 2023;49:998-1006. [Crossref] [PubMed]
- Bourdel N, Huchon C, Abdel Wahab C, et al. Borderline ovarian tumors: French guidelines from the CNGOF. Part 2. Surgical management, follow-up, hormone replacement therapy, fertility management and preservation. J Gynecol Obstet Hum Reprod 2021;50:101966. [Crossref] [PubMed]
- Wahab R, Hasan MM, Azam Z, et al. The role of coagulome in the tumor immune microenvironment. Adv Drug Deliv Rev 2023;200:115027. [Crossref] [PubMed]
- Kołodziejczyk J, Ponczek MB. The role of fibrinogen, fibrin and fibrin(ogen) degradation products (FDPs) in tumor progression. Contemp Oncol (Pozn) 2013;17:113-9. [Crossref] [PubMed]
- Zhang J, Chen J, Yang X, et al. Novel risk prediction models, involving coagulation, thromboelastography, stress response, and immune function indicators, for deep vein thrombosis after radical resection of cervical cancer and ovarian cancer. J Obstet Gynaecol 2023;43:2204162. [Crossref] [PubMed]
- Yang J, Wang C, Zhang Y, et al. Clinical significance and immune infiltration analyses of a novel coagulation-related signature in ovarian cancer. Cancer Cell Int 2023;23:232. [Crossref] [PubMed]
- Zhang C, Jia Y, Jia Y, et al. Prognostic and predictive value of plasma D-dimer levels in patients with small-cell lung cancer. Int J Clin Oncol 2018;23:1070-5. [Crossref] [PubMed]
- Lu S, Gong S, Wu F, et al. D-dimer to lymphocyte ratio can serve as a potential predictive and prognostic value in colorectal cancer patients with liver metastases. BMC Surg 2023;23:64. [Crossref] [PubMed]
- Gotta J, Gruenewald LD, Eichler K, et al. Unveiling the diagnostic enigma of D-dimer testing in cancer patients: Current evidence and areas of application. Eur J Clin Invest 2023;53:e14060. [Crossref] [PubMed]
- Zhang L, Guan Z, Yin Y, et al. Predictive value of indicator of CA125 combined with D-dimer (ICD) for lymph node metastasis in patients with ovarian cancer: A two center cohort study. J Cancer 2022;13:2447-56. [Crossref] [PubMed]
- Huchon C, Bourdel N, Abdel Wahab C, et al. Borderline ovarian tumors: French guidelines from the CNGOF. Part 1. Epidemiology, biopathology, imaging and biomarkers. J Gynecol Obstet Hum Reprod 2021;50:101965. [Crossref] [PubMed]
- Bourdel N, Huchon C, Abdel Wahab C, et al. Borderline ovarian tumors: Guidelines from the French national college of obstetricians and gynecologists (CNGOF). Eur J Obstet Gynecol Reprod Biol 2021;256:492-501. [Crossref] [PubMed]
- Guillaume A, Pirrello O. Preservation of fertility in surgery of benign and borderline malignant ovarian tumors. J Visc Surg 2018;155:S17-21. [Crossref] [PubMed]
- Wang P, Fang L. Salpingo-oophorectomy versus cystectomy in patients with borderline ovarian tumors: a systemic review and meta-analysis on postoperative recurrence and fertility. World J Surg Oncol 2021;19:132. [Crossref] [PubMed]
- Sobiczewski P, Piatek S, Michalski W, et al. Obstetric outcomes after conservative management of ovarian borderline tumors in women of reproductive age: A single center experience. Eur J Obstet Gynecol Reprod Biol 2022;269:126-31. [Crossref] [PubMed]
- Kose C, Korpe B, Tatlici TK, et al. Management of Borderline Ovarian Tumours: Tertiary Centre Experience from Turkey. J Coll Physicians Surg Pak 2023;33:1201-3. [PubMed]
- Vine MF, Ness RB, Calingaert B, et al. Types and duration of symptoms prior to diagnosis of invasive or borderline ovarian tumor. Gynecol Oncol 2001;83:466-71. [Crossref] [PubMed]
- Borrelli GM, de Mattos LA, Andres MP, et al. Role of Imaging Tools for the Diagnosis of Borderline Ovarian Tumors: A Systematic Review and Meta-Analysis. J Minim Invasive Gynecol 2017;24:353-63. [Crossref] [PubMed]
- Yazbek J, Raju KS, Ben-Nagi J, et al. Accuracy of ultrasound subjective 'pattern recognition' for the diagnosis of borderline ovarian tumors. Ultrasound Obstet Gynecol 2007;29:489-95. [Crossref] [PubMed]
- Yang S, Tang H, Xiao F, et al. Differentiation of borderline tumors from type I ovarian epithelial cancers on CT and MR imaging. Abdom Radiol (NY) 2020;45:3230-8. [Crossref] [PubMed]
- He X, Ying R, Jia L, et al. Retrospective study of characteristics and hyperthermia intraperitoneal perfusion in mucinous borderline ovarian tumor and mucinous ovarian carcinoma. Gland Surg 2023;12:453-64. [Crossref] [PubMed]
- Shin KH, Kim HH, Kwon BS, et al. Clinical Usefulness of Cancer Antigen (CA) 125, Human Epididymis 4, and CA72-4 Levels and Risk of Ovarian Malignancy Algorithm Values for Diagnosing Ovarian Tumors in Korean Patients With and Without Endometriosis. Ann Lab Med 2020;40:40-7. [Crossref] [PubMed]
- Xiao F, Zhang L, Yang S, et al. Quantitative analysis of the MRI features in the differentiation of benign, borderline, and malignant epithelial ovarian tumors. J Ovarian Res 2022;15:13. [Crossref] [PubMed]



