
Incidence, clinical features, and survival outcomes of primary malignant conjunctival tumor: a US population-based retrospective cohort analysis based on the SEER database (1975–2018)
Highlight box
Key findings
• The incidence rate of primary malignant conjunctival tumors (PMCT) increased after 1975 and decreased after 1997.
• Age ≥75 years, melanoma, and advanced stage were associated with worse Disease-specific survival (DSS).
• Age ≥75 years, male, unmarried status, and advanced stage were related to worse overall survival (OS). Lymphoma was related to better OS.
• Surgery may improve the prognosis of patient with PMCT.
What is known and what is new?
• PMCT is relatively uncommon and the epidemiological information is scare.
• This study described the epidemiological characteristics and successfully analyzed the risk factors for PMCT.
What is the implication, and what should change now?
• These findings are conducive to improving the management of patients with PMCT.
Introduction
The conjunctiva is a continuous mucous membrane consisted of the epithelium and stroma (1). However, because of their special anatomical sites and greater exposure to sunlight, conjunctival tumors are slightly different from tumors of other mucous membranes (2). The spectrum of conjunctival tumors varies considerably from benign to aggressive and potentially life-threatening malignancies. In a clinical study of 4,625 patients with conjunctival tumors, Shields et al. demonstrated that 52% of the tumors were benign, 18% were premalignant, and 30% were malignant (3). Among the malignancies, the most common types were melanoma, squamous cell carcinoma (SCC), and lymphoma. Other uncommon malignant tumor types, including malignant fibrous histiocytoma, Kaposi’s sarcoma, and metastatic tumors, have also been reported (3-6). Previous studies on conjunctival tumors classified tumors into histological types and specific diagnoses with relative frequencies and presented knowledge of the clinical characteristics of each tumor, which could help to establish a clinical diagnosis (3,7,8). However, epidemiological data on malignant conjunctival tumors, particularly primary malignant conjunctival tumors (PMCT), remain limited. Studying the epidemiology of PMCT can assist in assessing the public health burden associated with this disease. Additionally, it enables the identification of high-risk groups and facilitates the development of effective treatment strategies. Moreover, it enables clinicians to evaluate patient prognoses more accurately. The Surveillance, Epidemiology, and End Results (SEER) database involves about 30% of the total US population, and 48% of cancer patients in the US population (9,10). Based on the SEER program, Siegel et al. showed that the overall cancer incidence rate in the US has declined since the early 1990s, with a narrowing sex gap (11). Increasing regulation of smoking and advances in the early detection and comprehensive treatment of cancers have led to an overall lower mortality rate, decreasing from 215.1/100,000 in 1991 to 146.0/100,000 in 2019. In addition to incidence data, SEER also preserves annually uploaded data on patient demographics, date of diagnosis, tumor anatomic sites, tumor pathology, first course of therapeutic methods, stage at diagnosis, and follow-up vital status, acting as a tool for the study of cancers, especially uncommon ones (12,13). The SEER program conducts regular long-term follow-ups of patients to update information on patients’ vital status and cause of death. Using these data, researchers can obtain the survival outcomes of patients and analyze the prognosis of specific cancers. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1629/rc).
Methods
Data source and cohort selection
The data of patients with PMCT were extracted from the SEER database using SEER*Stat software (version 8.4.3) (14). More patients were enrolled, including those enrolled during 2000–2018 from SEER 18 Registries, patients diagnosed during 1992–1999 from SEER 13 Registries, and patients diagnosed during 1975–1991 from SEER 9 Registries (15-17). The inclusion criteria were as follows: (I) site-specific code C69.0 (conjunctiva) used to identify patients with first primary conjunctival malignancies, (II) active follow-up, and (III) microscopically confirmed diagnosis. The exclusion criteria were as follows: (I) diagnosis from autopsy or death certificate, (II) survival less than 1 month after diagnosis, and (III) unknown death information, laterality, or race. The following data were collected from the SEER database: age, year of diagnosis, sex, race, marital status, laterality, SEER stage, histological type, surgery, radiotherapy, chemotherapy, survival (months), cause of death, and vital status. Histological types were divided into four groups: SCC, melanoma, lymphoma, and others. The annual percentage changes (APCs) of PMCT from 1975 to 2020 were extracted from the SEER 8 Registries and the incidence rates were age-adjusted to the 2000 US standard population (18). The primary endpoints of this study were disease-specific survival (DSS) and overall survival (OS). DSS was calculated as the interval (months) between the diagnosis and death attributed to PMCT, and OS was calculated as the interval (months) between the diagnosis and death attributed to any cause. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
The age-adjusted incidence rates of PMCT were calculated per 100,000 persons using SEER*Stat software (version 8.4.3). APCs with 95% confidence intervals (CI) were calculated as well. The turning points of the incidence rate were calculated using Joinpoint software. Incidence rates were compared based on age, race, sex, and histological type. All continuous variables were converted into categorical variables and performed statistical analysis through the Chi-squared test or Fisher’s exact test. Survival curves were conducted through the Kaplan-Meier method and differences were analyzed by the log-rank test. The Cox proportional hazards regression model was used for the univariate and multivariate analyses. Significant variables (P<0.05) acquired in the univariate Cox regression model and treatment methods were analyzed using a multivariate Cox regression model to calculate the independent prognostic variables for DSS and OS. The results were demonstrated as hazard ratios (HR) with 95% CI. All statistical analyses were conducted utilizing SPSS 26.0 (IBM) and R software (version 4.3.2).
Results
Incidence of PMCT
The overall incidence of PMCT between 1975–2020 was 0.136 per 100,000 persons, with an APC of 0.929 (95% CI: 0.289–1.573, P<0.05). During the last 45 years, the incidence rate raised steadily before 1997 and declined slightly after peaking (Figure 1A and Figure S1). The incidence rate raised with age. The incidence rates in individuals aged <60, 60–74, and ≥75 years were 0.07, 0.39, and 0.605, respectively. The incidence rate was 0.176 in males and 0.105 in females, and the APC for females was 1.357 (95% CI: 0.536–2.185, P<0.05). Considering ethnicity, the incidence among whites (0.139) was higher than that among blacks (0.076). The incidence rates among American Indians, Alaskan natives, and Asian/Pacific islanders were 0.113. The overall incidence of SCC and melanoma was 0.043 and 0.033, respectively, with stable incidence trends (Figure 1B,1C). The overall incidence of lymphoma, the most frequent histological subtype, was 0.056, which increased steadily before 1998 and decreased slightly thereafter (Figure 1D and Figure S2). The detailed data can be seen in Table 1.

Table 1
| Characteristics | Incidence rate | APC |
|---|---|---|
| Overall | 0.136 | 0.929 (0.289 to 1.573)* |
| Age, years | ||
| <60 | 0.07 | 1.349 (0.448 to 2.258)* |
| 60–74 | 0.39 | NA |
| ≥75 | 0.605 | NA |
| Sex | ||
| Male | 0.176 | 0.287 (−0.465 to 1.044) |
| Female | 0.105 | 1.357 (0.536 to 2.185)* |
| Race | ||
| White | 0.139 | 0.924 (0.274 to 1.578)* |
| Black | 0.076 | NA |
| Others# | 0.113 | NA |
| Histological type | ||
| SCC | 0.043 | −0.21 (−0.962 to 0.548) |
| Melanoma | 0.033 | NA |
| Lymphoma | 0.056 | NA |
| Others | 0.004 | NA |
*, the APC is significantly different from zero (P<0.05). Others#, American Indian/Alaska Native, and Asian/Pacific Islander. APC, annual percentage change; SCC, squamous cell carcinoma.
Demographics of patients with PMCT
Up to 2,853 eligible patients who underwent PMCT were enrolled in this study. The general information of this study cohort is presented in Table 2. The mean age of the 2,853 patients was 61.25±17.18 years with a median age of 63 years, among which 1,205 (42.24%) were aged <60 years, 968 (33.93%) were aged between 60–74 years, and 680 (23.83%) were aged ≥75 years. This study included 1,678 males (58.82%) and 1,175 females (41.18%). Most patients were whites (86.37%), married (54.92%), and unilateral (95.44%). Based on the SEER stage, most patients (74.48%) were in the localized stage. The proportion of patients in the regional and distant stages was 7.33% and 3.26%, respectively. Concerning treatment modalities, more than half of patients with PMCT revived surgery (53.28%), the proportion were higher in patients with SCC (68.04%) and melanoma (69.7%). Only a fraction of patients received radiotherapy (28.11%) or chemotherapy (10.52%). The three major pathological types were lymphoma (39.64%), SCC (34.88%), and melanoma (21.98%). The mean age of patients with lymphoma was younger (57.79 years) than the overall mean age, whereas the mean age of patients with SCC was much older (64.87 years). More male patients (76.38%) had SCC. Compared with other pathological types, lymphomas were relatively prone to being bilateral (11.32%). The number of patients with lymphoma treated with radiotherapy (64.01%) was higher than the number of patients with other types. The proportion of patients with lymphoma who underwent surgery (30.24%) was lower than that of patients with other pathological types.
Table 2
| Variables | Total | SCC | Melanoma | Lymphoma | Others |
|---|---|---|---|---|---|
| Number of patients, n (%) | 2,853 | 995 (34.88%) | 627 (21.98%) | 1,131 (39.64%) | 100 (3.51%) |
| Age, years | |||||
| Mean (SD) | 61.25 (17.18) | 64.87 (15.33) | 61.19 (17.9) | 57.79 (17.43) | 64.8 (18.86) |
| Median [IQR] | 63 [50, 74] | 66 [55, 76] | 64 [50, 75.5] | 59 [46, 71] | 70 [50.75, 80.25] |
| <60 | 1,205 (42.24%) | 343 (34.47%) | 251 (40.03%) | 579 (51.19%) | 32 (32%) |
| 60–74 | 968 (33.93%) | 365 (36.68%) | 210 (33.49%) | 363 (32.1%) | 30 (30%) |
| ≥75 | 680 (23.83%) | 287 (28.84%) | 166 (26.48%) | 189 (16.71%) | 38 (38%) |
| Year of diagnosis | |||||
| 1975–1985 | 135 (4.73%) | 61 (6.13%) | 47 (7.5%) | 24 (2.12%) | 3 (3%) |
| 1986–1996 | 370 (12.97%) | 140 (14.07%) | 91 (14.51%) | 125 (11.05%) | 14 (14%) |
| 1997–2007 | 1,048 (36.73%) | 327 (32.86%) | 214 (34.13%) | 473 (41.82%) | 34 (34%) |
| 2008–2018 | 1,300 (45.57%) | 467 (46.93%) | 275 (43.86%) | 509 (45%) | 49 (49%) |
| Sex | |||||
| Male | 1,678 (58.82%) | 760 (76.38%) | 325 (51.83%) | 529 (46.77%) | 64 (64%) |
| Female | 1,175 (41.18%) | 235 (23.62%) | 302 (48.17%) | 602 (53.23%) | 36 (36%) |
| Race | |||||
| White | 2,464 (86.37%) | 918 (92.26%) | 582 (92.82%) | 877 (77.54%) | 87 (87%) |
| Black | 153 (5.36%) | 32 (3.22%) | 18 (2.87%) | 98 (8.66%) | 5 (5%) |
| Others# | 236 (8.27%) | 45 (4.52%) | 27 (4.31%) | 156 (13.79%) | 8 (8%) |
| Marital status | |||||
| Married | 1,567 (54.92%) | 545 (54.77%) | 333 (53.11%) | 646 (57.12%) | 43 (43%) |
| Unmarried | 898 (31.48%) | 269 (27.04%) | 203 (32.38%) | 384 (33.95%) | 42 (42%) |
| Unknown | 388 (13.6%) | 181 (18.19%) | 91 (14.51%) | 101 (8.93%) | 15 (15%) |
| Laterality | |||||
| Unilateral | 2,723 (95.44%) | 994 (99.9%) | 626 (99.84%) | 1003 (88.68%) | 100 (100%) |
| Bilateral | 130 (4.56%) | 1 (0.1%) | 1 (0.16%) | 128 (11.32%) | 0 (0%) |
| SEER stage | |||||
| Localized | 2,125 (74.48%) | 831 (83.52%) | 452 (72.09%) | 773 (68.35%) | 69 (69%) |
| Regional | 209 (7.33%) | 64 (6.43%) | 104 (16.59%) | 32 (2.83%) | 9 (9%) |
| Distant | 93 (3.26%) | 2 (0.2%) | 12 (1.91%) | 77 (6.81%) | 2 (2%) |
| Unknown | 426 (14.93%) | 98 (9.85%) | 59 (9.41%) | 249 (22.02%) | 20 (20%) |
| Surgery | |||||
| Performed | 1,520 (53.28%) | 677 (68.04%) | 437 (69.7%) | 342 (30.24%) | 64 (64%) |
| No/unknown | 1,333 (46.72%) | 318 (31.96%) | 190 (30.3%) | 789 (69.76%) | 36 (36%) |
| Radiotherapy | |||||
| Performed | 802 (28.11%) | 31 (3.12%) | 33 (5.26%) | 724 (64.01%) | 14 (14%) |
| No/unknown | 2,051 (71.89%) | 964 (96.88%) | 594 (94.74%) | 407 (35.99%) | 86 (86%) |
| Chemotherapy | |||||
| Performed | 300 (10.52%) | 67 (6.73%) | 66 (10.53%) | 154 (13.62%) | 13 (13%) |
| No/unknown | 2,553 (89.48%) | 928 (93.27%) | 561 (89.47%) | 977 (86.38%) | 87 (87%) |
Others#, American Indian/Alaska Native, and Asian/Pacific Islander. SCC, squamous cell carcinoma; SD, standard deviation; IQR, interquartile range; SEER, Surveillance, Epidemiology, and End Results.
Survival analysis
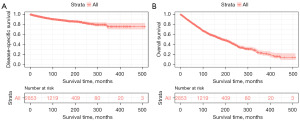
Kaplan–Meier analysis was conducted to evaluate DSS (Figure 2A) and OS (Figure 2B). A total of 1,107 patients died in the cohort, 254 of whom died of PMCT. The DSS rates at 5, 10, and 20 years were 94%, 89.6%, and 83.6%, respectively. The median OS was 118 months, and the OS rates at 5, 10, and 20 years were 80.9%, 63%, and 40.4%, respectively. Survival plots for age, year of diagnosis, sex, race, marital status, laterality, histological type, SEER stage, and treatment modality were plotted using Kaplan–Meier analysis. Survival outcomes improved over the years; the DSS for patients diagnosed in 2008–2018 was enhanced significantly compared to that of patients diagnosed in other periods (Figure 3A), while OS for patients diagnosed in 1997–2007 and 2008–2018 was better than that for patients diagnosed in 1975–1985 and 1986–1996 (Figure 3B). Survival time decreased with age, and patients aged over 75 had lower DSS (Figure 3C) and OS (Figure 3D) than younger patients. Males and females shared the same DSS (Figure 3E), whereas females tended to have better OS than males (Figure 3F). White people had worse DSS (Figure 3G) and OS (Figure 3H) than individuals of other races. Marital status had no impact on DSS, while married patients had better OS (Figure 4A,4B). Laterality had no impact on DSS, but bilateral lesions impacted OS (Figure 4C,4D). According to histological type, patients with conjunctival SCC had better DSS (Figure 4E), and patients with lymphoma had better OS (Figure 4F). Patients with local-stage lesions tended to have prolonged DSS and OS (Figure 4G,4H). Regarding treatment strategies, surgery was significantly related to better DSS (Figure 5A) but not OS (Figure 5B). Patients who underwent radiotherapy showed better DSS (Figure 5C) and OS (Figure 5D). However, chemotherapy did not influence DSS or OS (Figure 5E,5F).



Cox regression analysis was performed to discover the independent prognostic factors for both DSS and OS. Age, histological type, SEER stage, and surgery were all significantly related to DSS (Table 3). Multivariate Cox regression analysis showed that DSS was independently related to age (60–74 vs. <60 years, HR =2.033, 95% CI: 1.499–2.758, P<0.001; ≥75 vs. <60 years, HR =3.211, 95% CI: 2.309–4.466, P<0.001), histological type (melanoma vs. SCC, HR =4.637, 95% CI: 3.235–6.649, P<0.001; lymphoma vs. SCC, HR =1.141, 95% CI: 0.761–1.711, P=0.52; others vs. SCC, HR =2.055, 95% CI: 0.994–4.249, P=0.052), SEER stage (regional vs. localized, HR =1.55, 95% CI: 1.04–2.309, P=0.03; distant vs. localized, HR =4.318, 95% CI: 2.675–6.968, P<0.001; unknown vs. localized, HR =1.495, 95% CI: 1.072–2.085, P=0.02), and surgery status (performed vs. no/unknown, HR =1.565, 95% CI: 1.187–2.062, P=0.001). At the same time, age, sex, marital status, histological type, SEER stage, and surgery were significantly related to OS (Table 4). OS was independently related to age (60–74 vs. <60 years, HR =3.725, 95% CI: 3.139–4.421, P<0.001; ≥75 vs. <60 years, HR =9.399, 95% CI: 7.876–11.216, P<0.001), sex (female vs. male, HR =0.701, 95% CI: 0.612–0.803, P<0.001), marital status (unmarried vs. married, HR =1.342, 95% CI: 1.17–1.538, P<0.001, unknown vs. married, HR =1.204, 95% CI: 1.008–1.439, P=0.04), histological type (melanoma vs. SCC, HR =1.022, 95% CI: 0.873–1.197, P=0.78; lymphoma vs. SCC, HR =0.628, 95% CI: 0.533–0.74, P<0.001; others vs. SCC, HR =0.866, 95% CI: 0.633–1.184, P=0.37), SEER stage (regional vs. localized, HR =1.26, 95% CI: 1.017–1.561, P=0.04; distant vs. localized, HR =2.077, 95% CI: 1.498–2.881, P<0.001; unknown vs. localized, HR =1.114, 95% CI: 0.944–1.314, P=0.2), and surgery status (performed vs. no/unknown, HR =1.16, 95% CI: 1.018–1.322, P=0.03).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | <0.001 | <0.001 | |||
| <60 | Ref | Ref | |||
| 60–74 | 2.077 (1.537–2.807) | <0.001 | 2.033 (1.499–2.758) | <0.001 | |
| ≥75 | 3.331 (2.408–4.609) | <0.001 | 3.211 (2.309–4.466) | <0.001 | |
| Year of diagnosis | 0.003 | 0.68 | |||
| 1975–1985 | Ref | ||||
| 1986–1996 | 0.83 (0.529–1.303) | 0.42 | |||
| 1997–2007 | 0.57 (0.372–0.874) | 0.01 | |||
| 2008–2018 | 0.486 (0.303–0.78) | 0.003 | |||
| Sex | 0.59 | ||||
| Male | |||||
| Female | |||||
| Race | 0.03 | 0.28 | |||
| White | Ref | ||||
| Black | 0.742 (0.405–1.359) | 0.34 | |||
| Others# | 0.458 (0.25–0.839) | 0.01 | |||
| Marital status | 0.21 | ||||
| Married | |||||
| Unmarried | |||||
| Unknown | |||||
| Laterality | 0.69 | ||||
| Single side | |||||
| Bilateral | |||||
| Histological type | <0.001 | <0.001 | |||
| SCC | Ref | Ref | |||
| Melanoma | 4.723 (3.309–6.741) | <0.001 | 4.637 (3.235–6.649) | <0.001 | |
| Lymphoma | 1.425 (0.974–2.085) | 0.07 | 1.141 (0.761–1.711) | 0.52 | |
| Others | 2.42 (1.174–4.987) | 0.02 | 2.055 (0.994–4.249) | 0.052 | |
| SEER stage | <0.001 | <0.001 | |||
| Localized | Ref | Ref | |||
| Regional | 2.599 (1.763–3.83) | <0.001 | 1.55 (1.04–2.309) | 0.03 | |
| Distant | 3.556 (2.251–5.619) | <0.001 | 4.318 (2.675–6.968) | <0.001 | |
| Unknown | 1.376 (1.004–1.887) | 0.047 | 1.495 (1.072–2.085) | 0.02 | |
| Surgery | 0.02 | 0.001 | |||
| Performed | Ref | Ref | |||
| No/unknown | 1.345 (1.04–1.74) | 1.565 (1.187–2.062) | |||
| Radiotherapy | 0.03 | 0.23 | |||
| Performed | Ref | ||||
| No/unknown | 1.369 (1.028–1.823) | ||||
| Chemotherapy | 0.058 | 0.11 | |||
| Performed | |||||
| No/unknown | |||||
Others#, American Indian/Alaska Native, and Asian/Pacific Islander. DSS, disease-specific survival; HR, hazard ratio; CI, confidence interval; SCC, squamous cell carcinoma; SEER, Surveillance, Epidemiology, and End Results.
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | <0.001 | <0.001 | |||
| <60 | Ref | Ref | |||
| 60–74 | 3.833 (3.236–4.54) | <0.001 | 3.725 (3.139–4.421) | <0.001 | |
| ≥75 | 10.191 (8.57–12.118) | <0.001 | 9.399 (7.876–11.216) | <0.001 | |
| Year of diagnosis | <0.001 | 0.69 | |||
| 1975–1985 | Ref | ||||
| 1986–1996 | 1.01 (0.805–1.267) | 0.93 | |||
| 1997–2007 | 0.742 (0.597–0.923) | 0.007 | |||
| 2008–2018 | 0.73 (0.572–0.931) | 0.01 | |||
| Sex | <0.001 | <0.001 | |||
| Male | Ref | Ref | |||
| Female | 0.704 (0.622–0.797) | 0.701 (0.612–0.803) | |||
| Race | <0.001 | 0.13 | |||
| White | Ref | ||||
| Black | 0.665 (0.49–0.902) | 0.009 | |||
| Others# | 0.532 (0.407–0.696) | <0.001 | |||
| Marital status | <0.001 | <0.001 | |||
| Married | Ref | Ref | |||
| Unmarried | 1.354 (1.188–1.542) | <0.001 | 1.342 (1.17–1.538) | <0.001 | |
| Unknown | 1.373 (1.153–1.634) | <0.001 | 1.204 (1.008–1.439) | 0.04 | |
| Laterality | <0.001 | 0.09 | |||
| Single side | Ref | ||||
| Bilateral | 0.402 (0.27–0.598) | ||||
| Histological type | <0.001 | <0.001 | |||
| SCC | Ref | Ref | |||
| Melanoma | 0.878 (0.756–1.02) | 0.09 | 1.022 (0.873–1.197) | 0.78 | |
| Lymphoma | 0.496 (0.43–0.571) | <0.001 | 0.628 (0.533–0.74) | <0.001 | |
| Others | 1.025 (0.752–1.397) | 0.88 | 0.866 (0.633–1.184) | 0.37 | |
| SEER stage | <0.001 | <0.001 | |||
| Localized | Ref | Ref | |||
| Regional | 1.724 (1.399–2.125) | <0.001 | 1.26 (1.017–1.561) | 0.04 | |
| Distant | 1.351 (0.986–1.85) | 0.06 | 2.077 (1.498–2.881) | <0.001 | |
| Unknown | 0.94 (0.805–1.098) | 0.44 | 1.114 (0.944–1.314) | 0.20 | |
| Surgery | 0.43 | 0.03 | |||
| Performed | Ref | ||||
| No/unknown | 1.16 (1.018–1.322) | ||||
| Radiotherapy | <0.001 | 0.70 | |||
| Performed | Ref | ||||
| No/unknown | 1.755 (1.517–2.031) | ||||
| Chemotherapy | 0.35 | 0.86 | |||
| Performed | |||||
| No/unknown | |||||
Others#, American Indian/Alaska Native, and Asian/Pacific Islander. OS, overall survival; HR, hazard ratio; CI, confidence interval; SCC, squamous cell carcinoma; SEER, Surveillance.
Discussion
Due to its low incidence rate, few studies have estimated the epidemiology of PMCT and its impact on patient prognoses. This study provides a population-based cohort analysis to investigate the epidemiological characteristics and survival outcomes of patients with PMCT using the SEER program. Using the data, the incidence of PMCT was calculated and its clinicopathological characteristics, treatment outcomes, and prognostic factors were evaluated.
The results revealed that PMCT is an uncommon lesion with an overall incidence of 0.136 per 100,000 persons over the past 45 years. The incidence rate tended to increase relatively steadily before 1997, decreasing thereafter. Across different histological subtypes, the incidence trends of SCC and melanoma were steady over the four decades. Lymphoma, which had the highest incidence rate, increased before 1998, and slightly decreased thereafter. In a study of ocular and orbital lymphomas, Alfaar et al. reported an increasing trend in the incidence of ocular lymphomas from 1995 to 1997, followed by a steady decrease (19). The increasing trend in the incidence rate could be partly attributed to the development of diagnostic methods, modifications in the classification systems of non-Hodgkin’s lymphoma, and the increasing trend of acquired immunodeficiency syndrome (20,21). However, this decline could potentially be attributed to an inherent decrease in risk factors or a reduction in the ratio of solitary conjunctival lymphoma cases that are not related to other systemic lymphomas. The specific mechanisms underlying this pattern will require further investigation. Previous studies have shown that the incidence rate of conjunctival melanoma has been stable over time (22,23). Conjunctival SCC is the most common ocular surface malignancy in countries near the equator, with an increasing incidence rate, which may be due to human immunodeficiency virus infections and ultraviolet light exposure (24,25). In a study by Metekoua et al., the incidence of conjunctival SCC in South Africa was found to decrease between 2004 and 2014 (26). The different incidence trend patterns of conjunctival SCC in the present study may be partly explained by the latitude of the American and the relative low prevalence of human immunodeficiency virus. In this study, the incidence of PMCT was found to be higher in males than in females. In general, conjunctival lymphoma and conjunctival melanoma do not seem to have sex predilections (27-30). Conjunctival SCC predominantly affects the males in temperate countries, as the male gender may be more exposed to ultraviolet B radiation because of their preponderance in outdoor occupations (25,31). The incidence rate of PMCT increases with age, and the median diagnostic age in this study cohort was 63 years, which is in line with previous studies that reported that conjunctival SCC, melanoma, and lymphoma are the primary conjunctival tumors in the elderly population (2,27,30,32).
In contrast to other studies, the most common histological subtype of PMCT in this study was lymphoma, followed by SCC and melanoma. Conjunctival SCC was the most common conjunctival malignancy in a study conducted by Grossniklaus et al., whereas conjunctival melanoma accounted for the majority of the studies conducted by Shields et al. (3,8,33). Possible reasons for this difference include the identification of conjunctival SCC and corneal SCC and the increasing incidence of conjunctival lymphoma. Moreover, in the present study, different histological types of PMCT were demonstrated to have different survival outcomes. Conjunctival melanoma was associated with a worse DSS, whereas conjunctival lymphoma was associated with a better OS. Conjunctival melanoma is well known for its invasive nature. It can proliferate and disseminate on the ocular surface, including the cornea, and directly invade the globe, eyelids, orbit, nasolacrimal system and sinuses (34,35). Local recurrence was observed in 50% of all cases, and metastases were detected in 16–32% of patients (35). Conjunctival melanoma has a poor prognosis. The DSS rate of conjunctival melanoma at 10 years varies from 62% to 76% (22,36,37). A Dutch study conducted by Brouwer et al. demonstrated that initial treatment at a large referral center enhanced the clinical outcomes of patients with conjunctival melanoma (38). Thus, they recommended that these patients should be referred as early as possible. According to disease stage, our results demonstrated that the SEER stage was related to DSS and OS. Patients with distant SEER stages exhibited poor survival outcomes. Due to incomplete data on TNM in the SEER database, we could not obtain sufficient information to calculate the TNM stage of primary conjunctival malignant tumors. SEER stage is a standardized and simplified code that documents consistent tumor stage definitions over time (39). Most PMCT cases in our study were diagnosed at an early stage of the disease. In contrast to other mucous membranes throughout the body, the conjunctiva is readily observable. Due to the typical clinical features of conjunctival tumors, diagnoses are often achievable through routine ophthalmic examination and slit-lamp biomicroscopy performed by a skilled ophthalmologist. Consequently, tumors and associated lesions originating in the conjunctiva are typically detected at a comparatively early phase (40).
Surgical excision is the conventional therapy for the majority of cases of conjunctival malignancies. Except for lymphoma, surgery was the predominant intervention in patients with PMCT in our study. Surgery was also notably linked to prolonged DSS and OS. Incisional biopsy is performed for extensive suspicious malignancies, such as large melanoma, SCC, and primary acquired melanosis (2). However, incisional biopsy should be avoided for conjunctival melanoma, as it may increase the risk of recurrence (29,41). It is important to notice that if the conjunctival tumor occupies 4 hours or less on the bulbar or ≤15 mm basal dimension, excisional surgery and biopsy is usually preferable over incisional biopsy (42). In cases of SCC, epibulbar osseous choristoma, and melanoma, excisional biopsy is the preferred option to avoid inattentive tumor seeding. Complete excision and conjunctival reconstruction are feasible for tumors arising in the conjunctival fornix. However, most PMCTs, including SCC and melanoma, invade the intervertebral area near the limbus (8). Malignant limbal tumors probably invade the corneal epithelium and sclera that surround the tumor into the anterior chamber and disseminate through soft tissues into the orbit (35,43). Therefore, resecting a thin lamella of the sclera to obtain tumor-free margins could reduce the risk of tumor recurrence by avoiding friable tumor seeding into adjacent tissues. It is wise to use a delicate surgical technique, refraining from direct contact with the tumor, as well as to use microscopic techniques and keep a dry operative field to minimize the seeding of tumor cells (2).
In the present study, although radiotherapy was less common than surgery in patients with PMCT, more than half of the patients with conjunctival lymphoma received radiotherapy. Conjunctival tumors can be treated with two forms of radiotherapy: external beam radiotherapy and brachytherapy. External beam radiotherapy is often the preferred treatment option for conjunctival lymphoma, especially for lymphoma confined to the conjunctiva (27,44). A traditional radiation dosage of 20–30 Gy has been widely recommended for addressing such lesions, while studies also indicate that much lower doses may be sufficient (45,46). Brachytherapy directly sends a radioactive source to the tumor surface through various applicators, including plaques and seeds, and is used after conjunctival epithelial healing from lesion resection (47). Generally, brachytherapy is employed for patients with diffuse tumors that cannot be resected completely, and those with multiple recurrences (2). Increasing evidence demonstrates that topical chemotherapy, which has the potential to eliminate subclinical tumor cells, is a prominent therapy for conjunctival tumors, both in combination with surgical procedures and as a standalone therapy (48,49). It is preferable for diffuse, multifocal tumors invading the cornea and limbus, or when surgical resection margins test positive. However, owing to the relative rarity of PMCT, which limits the conducting of comparable studies, at present no standard guidelines exist for the utilization of diverse drugs across different types of conjunctival tumors.
There are several limitations in this study. First, as this was a retrospective study, bias was inevitable. Although we enrolled as many eligible patients as possible, the relationship between risk factors and prognosis may not have been well-identified due to the insufficient number of patients in the Cox regression model. However, to the best of our knowledge, the number of patients with PMCT in this study is the highest yet to be evaluated in the literature. Second, many factors that could impact survival outcomes are not available in the SEER database, including various laboratory indicators, vision preservation, and some potential prognostic variables could not be included. Third, the patients enrolled in our study may be unrepresentative, owing to an imbalance in race distribution in the SEER database. Further research based on different regions and races is required to obtain generalized results. Despite these limitations, the SEER database remains a reliable and valuable resource for researching uncommon malignancies.
Conclusions
The findings presented in this study indicate that PMCT is relatively uncommon, with an increasing incidence rate after 1975 up until 1997, followed by a slightly decreasing trend. Age, histological type, SEER stage, and surgery were all significantly associated with DSS and OS, while age ≥75 years, melanoma, and distant SEER stage were found to potentially lead to worse DSS. Similarly, age ≥75 years, male, unmarried status, and distant SEER stage were associated with worse OS, while lymphoma was related to better OS. Surgery is likely to improve the survival outcomes of patients. Taken together, these results provide a new perspective for improving the management and healthcare of patients with PMCT.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1629/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1629/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1629/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chi MJ, Baek SH. Clinical analysis of benign eyelid and conjunctival tumors. Ophthalmologica 2006;220:43-51. [Crossref] [PubMed]
- Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol 2019;67:1930-48. [Crossref] [PubMed]
- Shields CL, Alset AE, Boal NS, et al. Conjunctival Tumors in 5002 Cases. Comparative Analysis of Benign Versus Malignant Counterparts. The 2016 James D. Allen Lecture. Am J Ophthalmol 2017;173:106-33. [Crossref] [PubMed]
- Coblentz J, Park JY, Discepola G, et al. Conjunctival Kaposi's sarcoma with orbital extension in an HIV-negative man. Can J Ophthalmol 2018;53:e111-3. [Crossref] [PubMed]
- Kiratli H, Shields CL, Shields JA, et al. Metastatic tumours to the conjunctiva: report of 10 cases. Br J Ophthalmol 1996;80:5-8. [Crossref] [PubMed]
- Arora R, Monga S, Mehta DK, et al. Malignant fibrous histiocytoma of the conjunctiva. Clin Exp Ophthalmol 2006;34:275-8. [Crossref] [PubMed]
- Shields CL, Sioufi K, Alset AE, et al. Clinical Features Differentiating Benign From Malignant Conjunctival Tumors in Children. JAMA Ophthalmol 2017;135:215-24. [Crossref] [PubMed]
- Shields CL, Demirci H, Karatza E, et al. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology 2004;111:1747-54. [Crossref] [PubMed]
- Che WQ, Li YJ, Tsang CK, et al. How to use the Surveillance, Epidemiology, and End Results (SEER) data: research design and methodology. Mil Med Res 2023;10:50. [Crossref] [PubMed]
- Guo X, Wang L, Beeraka NM, et al. Incidence Trends, Clinicopathologic Characteristics, and Overall Survival Prediction in Retinoblastoma Children: SEER Prognostic Nomogram Analysis. Oncologist 2024;29:e275-81. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Zhang M, Xiao F, Lin M, et al. The epidemiology and prognosis of patients with primary gastric T-cell lymphoma in the SEER program. Cancer Med 2023;12:84-98. [Crossref] [PubMed]
- Peng F, Su W, Zhang A, et al. Investigation of epidemiological characteristics and development of a nomogram to predict survival in primary ocular adnexal lymphoma. Clin Exp Ophthalmol 2022;50:615-31. [Crossref] [PubMed]
- Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.4.3.
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000-2018) - Linked To County Attributes - Total U.S., 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission.
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER Research Plus Data, 13 Registries, Nov 2020 Sub (1992-2018) - Linked To County Attributes - Total U.S., 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission.
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER Research Plus Data, 9 Registries, Nov 2020 Sub (1975-2018) - Linked To County Attributes - Total U.S., 1969-2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission.
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER Research Data, 8 Registries, Nov 2022 Sub (1975-2020) - Linked To County Attributes - Time Dependent (1990-2021) Income/Rurality, 1969-2021 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission.
- Alfaar AS, Yousef YA, W, Wilson M, et al. Declining incidence and improving survival of ocular and orbital lymphomas in the US between 1995 and 2018. Sci Rep 2024;14:7886. [Crossref] [PubMed]
- Moslehi R, Coles FB, Schymura MJ. Descriptive epidemiology of ophthalmic and ocular adnexal non-Hodgkin's lymphoma. Expert Rev Ophthalmol 2011;6:175-80. [Crossref] [PubMed]
- Eltom MA, Jemal A, Mbulaiteye SM, et al. Trends in Kaposi's sarcoma and non-Hodgkin's lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst 2002;94:1204-10. [Crossref] [PubMed]
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol 1996;234:569-72. [Crossref] [PubMed]
- Seregard S, Kock E. Conjunctival malignant melanoma in Sweden 1969-91. Acta Ophthalmol (Copenh) 1992;70:289-96. [Crossref] [PubMed]
- Santoni A, Thariat J, Maschi C, et al. Management of Invasive Squamous Cell Carcinomas of the Conjunctiva. Am J Ophthalmol 2019;200:1-9. [Crossref] [PubMed]
- Gichuhi S, Ohnuma S, Sagoo MS, et al. Pathophysiology of ocular surface squamous neoplasia. Exp Eye Res 2014;129:172-82. [Crossref] [PubMed]
- Metekoua C, Ruffieux Y, Olago V, et al. Decreasing incidence of conjunctival squamous cell carcinoma in people with HIV in South Africa. J Natl Cancer Inst 2023;115:1213-9. [Crossref] [PubMed]
- Kirkegaard MM, Coupland SE, Prause JU, et al. Malignant lymphoma of the conjunctiva. Surv Ophthalmol 2015;60:444-58. [Crossref] [PubMed]
- Kirkegaard MM, Rasmussen PK, Coupland SE, et al. Conjunctival Lymphoma--An International Multicenter Retrospective Study. JAMA Ophthalmol 2016;134:406-14. [Crossref] [PubMed]
- Larsen AC. Conjunctival malignant melanoma in Denmark: epidemiology, treatment and prognosis with special emphasis on tumorigenesis and genetic profile. Acta Ophthalmol 2016;94 Thesis 1:1-27.
- Virgili G, Parravano M, Gatta G, et al. Incidence and Survival of Patients With Conjunctival Melanoma in Europe. JAMA Ophthalmol 2020;138:601-8. [Crossref] [PubMed]
- Hӧllhumer R, Williams S, Michelow P. Ocular surface squamous neoplasia: management and outcomes. Eye (Lond) 2021;35:1562-73. [Crossref] [PubMed]
- Ramberg I, Heegaard S, Prause JU, et al. Squamous cell dysplasia and carcinoma of the conjunctiva. A nationwide, retrospective, epidemiological study of Danish patients. Acta Ophthalmol 2015;93:663-6. [Crossref] [PubMed]
- Grossniklaus HE, Green WR, Luckenbach M, et al. Conjunctival lesions in adults. A clinical and histopathologic review. Cornea 1987;6:78-116. [Crossref] [PubMed]
- Kaštelan S, Gverović Antunica A, Beketić Orešković L, et al. Conjunctival Melanoma - Epidemiological Trends and Features. Pathol Oncol Res 2018;24:787-96. [Crossref] [PubMed]
- Vora GK, Demirci H, Marr B, et al. Advances in the management of conjunctival melanoma. Surv Ophthalmol 2017;62:26-42. [Crossref] [PubMed]
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci 2002;43:3399-408.
- Lommatzsch PK, Lommatzsch RE, Kirsch I, et al. Therapeutic outcome of patients suffering from malignant melanomas of the conjunctiva. Br J Ophthalmol 1990;74:615-9. [Crossref] [PubMed]
- Brouwer NJ, Marinkovic M, van Duinen SG, et al. Treatment of conjunctival melanoma in a Dutch referral centre. Br J Ophthalmol 2018;102:1277-82. [Crossref] [PubMed]
- Wang SJ, Emery R, Fuller CD, et al. Conditional survival in gastric cancer: a SEER database analysis. Gastric Cancer 2007;10:153-8. [Crossref] [PubMed]
- Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Surv Ophthalmol 2004;49:3-24. [Crossref] [PubMed]
- Larsen AC, Dahmcke CM, Dahl C, et al. A Retrospective Review of Conjunctival Melanoma Presentation, Treatment, and Outcome and an Investigation of Features Associated With BRAF Mutations. JAMA Ophthalmol 2015;133:1295-303. [Crossref] [PubMed]
- Honavar SG, Manjandavida FP. Tumors of the ocular surface: A review. Indian J Ophthalmol 2015;63:187-203. [Crossref] [PubMed]
- Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea 2003;22:687-704. [Crossref] [PubMed]
- McGrath LA, Ryan DA, Warrier SK, et al. Conjunctival Lymphoma. Eye (Lond) 2023;37:837-48. [Crossref] [PubMed]
- Lee GI, Oh D, Kim WS, et al. Low-Dose Radiation Therapy for Primary Conjunctival Marginal Zone B-Cell Lymphoma. Cancer Res Treat 2018;50:575-81. [Crossref] [PubMed]
- Pinnix CC, Dabaja BS, Milgrom SA, et al. Ultra-low-dose radiotherapy for definitive management of ocular adnexal B-cell lymphoma. Head Neck 2017;39:1095-100. [Crossref] [PubMed]
- Stannard C, Sauerwein W, Maree G, et al. Radiotherapy for ocular tumours. Eye (Lond) 2013;27:119-27. [Crossref] [PubMed]
- Kim JW, Abramson DH. Topical treatment options for conjunctival neoplasms. Clin Ophthalmol 2008;2:503-15. [Crossref] [PubMed]
- Midena E, Frizziero L, Parrozzani R. Pharmacotherapy and Immunotherapy of Conjunctival Tumors. Asia Pac J Ophthalmol (Phila) 2017;6:121-31. [Crossref] [PubMed]


