
Endobronchial malignancy as a manifestation of advanced ovarian cancer recurrence: a case report and literature review
Highlight box
Key findings
• Flexible bronchoscopy combined with imaging and immunohistochemistry tests are efficient to make a diagnosis of endobronchial metastatic ovarian cancer (OC).
• Endobronchial intervention, radiotherapy, and chemotherapy are potentially efficient treatment modalities for OC patients with endobronchial metastasis.
• The prognosis of those with multiple metastasis appears to be poor.
What is known and what is new?
• Endobronchial metastasis from primary OC is very rare.
• A more favourable prognosis may be achieved for OC patients with endobronchial metastasis following combined comprehensive therapies.
What is the implication, and what should change now?
• Endobronchial metastasis could be suspected in ovarian carcinoma patients who develop respiratory symptoms. They should undergo additional imaging and be considered for flexible bronchoscopy.
Introduction
Ovarian cancer (OC) ranks third amongst gynecologic malignancies worldwide, with 313,959 new diagnoses and 207,252 deaths globally in 2020 (1). Recognized as the most lethal gynecologic malignancy, OC poses diagnostic challenges due to the absence of specific symptoms (2). Consequently, most OC patients are diagnosed at an advanced stage with most common metastatic sites being the omentum, and the peritoneum (3). Endobronchial metastasis from primary OC is extremely rare in clinical practice. From the first case of endobronchial metastasis from primary OC reported by Westerman in 1980, there have been only 12 reported cases until now (4-6). Given the long latent period and the atypical clinical characteristics, discerning it from a primary bronchial tumor poses a considerable challenge. Here, we present a case illustrating endobronchial metastatic recurrence originating from primary OC. We present this case in accordance with the CARE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-507/rc).
Case presentation
A 51-year-old female patient presented with a 3-month history of intermittent cough, expectoration, and suffocating pneumonia. Symptoms included chest tightness, right-sided back pain, weakness, and night sweats in the preceding 3 months. A chest computed tomography (CT) scan revealed multiple nodular masses in the right upper lobe, soft tissue thickening with bronchial invasion in the left upper lobe, and enlargement of bilateral hilar and mediastinal lymph nodes. Despite treatment with moxifloxacin, doxofylline, and hydrocortisone, her symptoms persisted, leading to admission to Tianjin Chest Hospital in November 2018.
She was diagnosed in July 2016 with an International Federation of Gynecology and Obstetrics (FIGO) stage 4B OC, owing to metastatic disease in the supraclavicular lymph nodes. The patient initially underwent one cycle of chemotherapy with taxol and oxaliplatin. Subsequently, in August 2016, she underwent surgical resection of the ovaries, uterus, omentum, and left supraclavicular lymph nodes. Post-resection, the patient received five cycles of chemotherapy with taxol and oxaliplatin, followed by additional five cycles with irinotecan and oxaliplatin until September 2017. From that point onward, she opted for traditional Chinese medicine as adjuvant therapy. Her progression-free survival was 27 months.
On physical examination, the patient displayed consciousness, a flat and soft abdomen without tenderness, clubbing of the fingers, and leg edema. Elevated carcinoembryonic antigen levels in serum were noted, along with other abnormal routine test results (Table 1). A chest X-ray revealed enlarged right and left upper hilar, spreading mediastinum, elevated right septum, and multiple nodular masses in the right upper lobe (Figure 1). Unfortunately, the patient’s condition is critical. Therefore, she did not have a chest CT scan. To differentiate between primary tumor and metastasis, a flexible bronchoscopy was performed. The mucosa appeared congested and oedematous, with broadening of the tracheal carina (Figure 2A). A granulation mass projected from the opening wall of the right main bronchus (Figure 2B), and stenosis of the right main bronchus and apical, middle, and posterior segmental bronchi in the right lobe were observed (Figure 2C). The opening of the left main bronchus was nearly completely obstructed by a visible neoplasm (Figure 2D). Further observation involved removing the neoplasm using a clip and electric needle knife, revealing thickened and uneven mucosa. We did not perform endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in order to alleviate the patient’s respiratory obstruction symptoms and reduce the risk of prolonged operation. Hematoxylin-eosin (HE) staining indicated tightly arranged tumor cells with irregular shapes (Figure 3A), showing evident atypia upon magnification (Figure 3B). Immunohistochemistry staining displayed anti-pan cytokeratin (CK) positivity in the cytoplasm and anti-Wilms tumor protein 1 (WT-1) positivity in the nucleus (Figure 4A,4B). Additionally, the neoplasm exhibited positive expression of p53, and the rate of Ki-67 expression exceeded 70% (Table 2). Integrating these findings, the pathological diagnosis was endobronchial tumor recurrence from primary OC. The masses at the openings of the left main bronchi (Figure 2D) and right main bronchi (Figure 2B) are considered endobronchial metastatic recurrence. Due to intolerance, the patient did not pursue further chemotherapy. Rapid disease progression ensued, leading the patient to abandon treatment, and she passed away in December 2018.
Table 1
| Index | Value |
|---|---|
| Blood routine texting | |
| Hb | 126 g/L |
| Fg | 5.35 g/L |
| ESR | 49.0 mm/h |
| Neu | 72.10% |
| PLT | 337.00×109/L |
| D-dimer | 1.00 µg/mL |
| WBC | 5.29×109/L |
| Arterial blood gas | |
| pCO2 | 37.0 mmHg |
| cHCO3− | 27.4 mmol/L |
| FiO2 | 29.0% |
| pH | 7.472 |
| pO2 | 69.0 mmHg |
| sO2 | 94.70% |
| Tumor makers | |
| CYFRA21-1 | 18.57 ng/mL |
| NSE | 58.07 ng/mL |
| CA125 | 86.82 U/mL |
| Echocardiography | |
| PAP | 30 mmHg |
| RVAW | 3 mm |
| EF | 56% |
| RVOT | 28 mm |
CA, cancer antigen; EF, ejection fraction; ESR, erythrocyte sedimentation rate; Fg, fibrinogen; Hb, hemoglobin; Neu, neutrophils; NSE, neuron-specific enolase; PAP, pulmonary artery pressure; PLT, platelet; RVAW, right ventricular anterior wall; RVOT, right ventricular outflow tract; WBC, white blood cell.
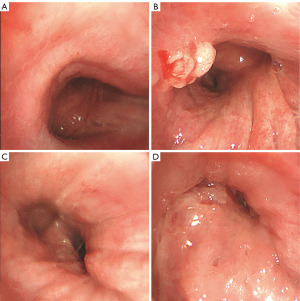
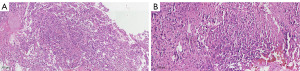
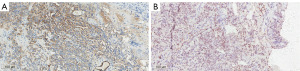
Table 2
| Antibody | Carcinomatous component |
|---|---|
| ER | − |
| WT-1 | + |
| Napsin A | − |
| CK | + |
| P40 | − |
| Ki-67 | >70% |
| PR | − |
| TTF-1 | − |
| CD56 | − |
| P53 | + |
| PAS | − |
−, negative; +, positive. CK, cytokeratin; ER, estrogen receptor; PAS, periodic acid Schiff; PR, progesterone receptor; TTF-1, transcriptional regulatory factor-1; WT-1, Wilms tumor protein 1.
A literature search was conducted in http://med.wanfangdata.com.cn and http://www.ncbi.nlm.nih.gov/pubmed. A total of 11 papers published in English with no papers in Chinese have previously reported 12 cases who experienced endobronchial metastasis from primary OC to date (Table 3) (4-14).
Table 3
| First author | Age, years | Type | Time (years) | Imaging examination | Clinical manifestations | Therapy | Prognosis | |
|---|---|---|---|---|---|---|---|---|
| Metastasis to other sites | Survival (months) | |||||||
| Westerman (4) | 51 | PC | 7 | Compact shadow in trachea | Dyspnea | Radiotherapy | NR | NR |
| Merrill (5) | 45 | SCC | 12 | Compact shadow in right middle lobe | Cough | Right lower lobe resection | NR | NR |
| Merimsky (6) | 83 | PA | 0.2 | Airway obstruction | Dyspnea | Laser therapy | Abdomen | 4 |
| Mateo (7) | 62 | SA | 5 | Bronchial obstruction | Dyspnea, cough | Chemotherapy + radiotherapy | Brain | 22 |
| Wholey (8) | 49 | NR | 2 | Bronchial obstruction | Dyspnea | NR | NR | NR |
| Petru (9) | 40 | SPA | 2.7 | Lymphadenovarix | Dyspnea | Laser therapy + chemotherapy | NR | 6 |
| Choi (10) | 33 | SPA | 7 | Airway obstruction | Dyspnea, hemoptysis | Chemotherapy + radiotherapy | No | >66 |
| Harrington (11) | 42 | SPA | 0.92 | Right middle lobe atelectasis | Hemoptysis | Chemotherapy + radiotherapy chemotherapy + radiotherapy | NR | NR |
| Upadhyay (12) | 62 | SA | 21 | NR | Dyspnea, cough | Chemotherapy | No | >18 |
| Upadhyay (12) | 53 | SA | 6.3 | NR | Dyspnea, cough | Electric needle knife | NR | 18 |
| Dhillon (13) | 61 | SPA | 12 | Lymphadenovarix and calcification | Hemoptysis | Radiotherapy + laser therapy | No | >36 |
| Ayub (14) | 22 | PA | 2 | Bronchial obstruction | Hemoptysis | No | NR | 6 |
EC, endobronchial; NR, none reported; PA, papillary adenocarcinoma; PC, papillary cystadenocarcinoma; SA, serous adenocarcinoma; SCC, serous cystadenocarcinoma; SPA, serous papillary adenocarcinoma; Time, time metastasis to EC.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Discussion among physicians from Tianjin Chest Hospital
We conducted interdisciplinary and multidisciplinary consultations for this case. OC is recognized as the most lethal gynecologic malignancy, with OC cells capable of directly invading neighboring pelvic organs such as the bladder (17%) and rectosigmoid or migrating to more distant organs such as the peritoneum and omentum (86%), intestines (50%), and spleen (20%) through peritoneal fluid transportation (15). Other metastatic routes include lymphatics with hematogenous metastasis accounting for only 16%. Hematogenous metastatic sites typically involve the liver, lungs, and pleura (15). Although end-stage OC can metastasize to the lungs, endobronchial metastasis is extremely rare, with no prior research conducted on this disease in China prior to our study. We speculate a minimal residual prevalence of supraclavicular lymph nodes following initial OC cytoreduction as a trigger for this exceedingly rare endobronchial recurrence.
The intervals between primary OC and an endobronchial recurrence are consistently long, with a mean interval of 65.3 months and a maximum interval of up to 21 years (12). The progression to an endobronchial metastasis from primary OC is gradual, and the median survival time of 10 cases before 2018 ranged from 6 to 24 months (16). Compared with metastatic chest tumors, endobronchial metastasis from primary OC often exhibits a favorable prognosis (17). Tumor stage plays a pivotal role in determining the prognosis of such patients. Franco et al. (18) reported a case of an OC patient at stage IA with endobronchial recurrence who underwent left lower lobectomy followed by chemotherapy, showing no evidence of disease activity during the five-year follow-up.
Department of Radiology
The potential mechanisms of endobronchial metastasis from primary OC are intricate and may include mediastinal lymph node and haematogenous parenchymal metastatic patterns (9,11,19). Routine imaging examinations often fail to distinguish endobronchial metastasis from primary bronchial tumors. Clinical manifestations of endobronchial metastases include dyspnea, dry cough, hemoptysis, anhelation, and hoarseness. However, 52–62.5% of patients show no respiratory symptoms (19). Chest CT may reveal airway stenosis and thickening of vessel walls, which may be attributed to intratracheal, tracheal mucosa, and airway surroundings diseases. Unfortunately, because early airway metastasis is caused by nodular or chronic invasion and early chest CT scans may be normal (CT scans may not show early changes, so some patients may not have airway lesions), only 50% of endobronchial diseases are detectable by chest CT, leading to frequent misdiagnosis of endobronchial metastasis from primary OC as primary bronchial tumors (5).
Department of Respiratory and Critical Care Medicine
Flexible bronchoscopy serves as a direct detection method for endobronchial metastasis, revealing a variety of features, including nodular masses combined with necrosis. However, it falls short in differentiating between benign tumors, primary lung cancer, and tumor metastasis. Pathological and immunohistochemistry assays become imperative to identify the origins of the tumors. According to metastatic modes, tumors metastasizing to airways can be categorized into four types: type I (direct metastatic tumor), type II (airway tumors invaded from pulmonary solid lesions), type III (airway tumors invaded from lymph nodes of the mediastinum and hilum), and type IV (airway tumors invaded from peripheral lesions) (20). Types II and III predominate. The bronchoscopy image of the patient clearly shows the subcutaneous and exogenous invasion of cancer. We consider the coexistence of mediastinal lymph node invasion and bronchial metastasis. Dhillon et al. (13) reported a unique endobronchial metastasis combined with airway calcification, where clinical manifestations resembling airway calcification and broncholithiasis lead to misdiagnosis. Ayub et al. described an unusual endobronchial metastasis combined with aspergillosis, resulting in hemoptysis (14). Himeji et al. (21) performed tumor ablation and airway stenting using a hybrid stent on a patient with upper tracheal stenosis caused by endobronchial metastasis of OC. Six months after placing the stent, the patient remained alive, with no reported stent migration or bleeding, highlighting the significant role of flexible bronchoscopy in the diagnosis and treatment of OC patients with endobronchial metastasis.
Department of Oncology
The choice of treatments for endobronchial metastasis, including resection, chemotherapy, and radiotherapy, relies on various factors such as patient status, age, tumor size, location, and other considerations. Choi et al. firstly reported the application of a needle electrical knife for removing bronchial metastatic foci, demonstrating effective relief for patients experiencing dyspnea and hemoptysis (10). Endobronchial interventions, including stents, local radiotherapy, and photodynamic therapy, may efficiently alleviate symptoms like dyspnea, hemoptysis, and stenosis induced by endobronchial metastasis. However, endobronchial interventions may display limited efficacy for patients with submucosal metastasis. Research suggests that atomizing chemotherapy could be an optimal method for submucosal metastasis due to prolonged detention and high drug concentration in lesions, estimating nearly 5–15 times higher drug concentration in tumor tissues compared to normal lung tissues (19). Novel therapeutic regimens are urgently required for the management of endobronchial metastases.
Conclusions
Flexible bronchoscopy combined with imaging and immunohistochemistry assays prove to be an effective diagnostic for identifying endobronchial metastasis in recurrent OC patients. Endobronchial interventions, such as ablations and airway stenting, along with radiotherapy, and chemotherapy emerge as efficient treatments for these patients. Endobronchial metastasis as a manifestation of advanced OC recurrence still carries an unfavorable prognosis and is indirectly related to the advanced stage of the primary disease.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-507/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-507/prf
Funding: This study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-507/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Richardson DL, Eskander RN, O'Malley DM. Advances in Ovarian Cancer Care and Unmet Treatment Needs for Patients With Platinum Resistance: A Narrative Review. JAMA Oncol 2023;9:851-9. [Crossref] [PubMed]
- Shimizu A, Lengyel E. Decoding evolutionary trajectories of ovarian cancer metastasis. Cancer Cell 2023;41:1008-10. [Crossref] [PubMed]
- Westerman DE, Urbanetti JS, Rudders RA, et al. Metastatic endotracheal tumor from ovarian carcinoma. Chest 1980;77:798-800. [Crossref] [PubMed]
- Merrill CR, Hopkirk JA. Late endobronchial metastasis from ovarian tumour. Br J Dis Chest 1982;76:253-4. [Crossref] [PubMed]
- Merimsky O, Greif J, Chaitchik S, et al. Endobronchial metastasis of ovarian cancer. A case report. Tumori 1990;76:614-5. [Crossref] [PubMed]
- Mateo F, Serur E, Smith PR. Bronchial metastases from ovarian carcinoma. Report of a case and review of the literature. Gynecol Oncol 1992;46:235-8. [Crossref] [PubMed]
- Wholey MH, Meyerrose GE, McGuire WP, et al. Endobronchial lesion from metastatic ovarian carcinoma resulting in partial right mainstem obstruction demonstrated by lung scintigraphy. Clin Nucl Med 1995;20:465-6. [Crossref] [PubMed]
- Petru E, Friedrich G, Pickel H, et al. Life-threatening tracheal metastasis complicating ovarian cancer--a case report. Gynecol Oncol 1999;74:141-2. [Crossref] [PubMed]
- Choi HS, Kim SY, Choi CW, et al. Use of bronchoscopic electrocautery in removing an endotracheal metastasis. Lung Cancer 2007;58:286-90. [Crossref] [PubMed]
- Harrington A, Mahrer T, Chang D. A case of ovarian carcinoma with endobronchial metastases. Chest 2011;140:29A. [Crossref]
- Upadhyay A, Goel V, Batra U, et al. Two cases of ovarian carcinoma with endobronchial metastases: Rare presentation. South Asian J Cancer 2015;4:149. [Crossref] [PubMed]
- Dhillon SS, Harris K, Pokharel S, et al. Calcified Mediastinal Metastasis of Ovarian Cancer Mimicking Broncholithiasis. J Bronchology Interv Pulmonol 2016;23:229-31. [Crossref] [PubMed]
- Ayub II, Thangaswamy D, Joseph LD, et al. Lung Parenchymal and Endobronchial Metastases From Ovarian Carcinoma. J Bronchology Interv Pulmonol 2018;25:235-8. [Crossref] [PubMed]
- Kerr VE, Cadman E. Pulmonary metastases in ovarian cancer. Analysis of 357 patients. Cancer 1985;56:1209-13. [Crossref] [PubMed]
- Thomakos N, Diakosavvas M, Machairiotis N, et al. Rare Distant Metastatic Disease of Ovarian and Peritoneal Carcinomatosis: A Review of the Literature. Cancers (Basel) 2019;11:1044. [Crossref] [PubMed]
- Dauplat J, Hacker NF, Nieberg RK, et al. Distant metastases in epithelial ovarian carcinoma. Cancer 1987;60:1561-6. [Crossref] [PubMed]
- Franco RM, Guimaraes MD, Moreira BL, et al. Enhancing survival with early surgical resection of endobronchial metastasis in a follow-up of ovarian carcinoma. Radiol Bras 2015;48:130. [Crossref] [PubMed]
- Pickel H, Lahousen M, Stettner H, et al. The spread of ovarian cancer. Baillieres Clin Obstet Gynaecol 1989;3:3-12. [Crossref] [PubMed]
- Kiryu T, Hoshi H, Matsui E, et al. Endotracheal/endobronchial metastases: clinicopathologic study with special reference to developmental modes. Chest 2001;119:768-75. [Crossref] [PubMed]
- Himeji D, Tanaka GI, Fukuyama C, et al. Endobronchial metastasis of ovarian cancer rescued by tumor ablation and a self-expanding hybrid stent: A case report and review of the literature. Respir Med Case Rep 2020;30:101132. [Crossref] [PubMed]



