
GJB2 enhances cancer stem cell properties by modulating SOX2 expression via NF-κB pathway activation in lung adenocarcinoma
Highlight box
Key findings
• Gap junction beta-2 protein (GJB2) is highly expressed in lung adenocarcinoma (LUAD) and regulates the NF-κB/SOX2 axis to promote cancer stem cell (CSC) properties in LUAD cells.
What is known and what is new?
• LUAD is a common type of lung cancer with a high prevalent and high mortality. GJB2 is closely related to the occurrence and development of cancer.
• We have revealed a novel mechanism by which GJB2 promotes the CSC properties of LUAD.
What is the implication, and what should change now?
• The results of this study provide possible molecular targets for the treatment of LUAD.
Introduction
Lung cancer is one of the most prevalent cancers globally and remains the leading cause of cancer-related mortality, representing a significant socioeconomic challenge (1). It is primarily categorized into two major pathological types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), with NSCLC constituting approximately 80% of all cases. NSCLC is further divided into lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) (2), with LUAD accounting for approximately 45% of lung cancer cases, a statistic that continues to rise early (3). While early-stage LUAD can be effectively treated through radical resection, advanced LUAD presents a therapeutic challenge due to limited treatment options. The poor outcomes in such cases are primarily attributed to the insufficient understanding of the underlying biological mechanisms driving the disease. Therefore, there is growing interest in identifying novel diagnostic approaches and uncovering the tumor’s pathogenesis.
Gap junction beta-2 protein (GJB2), or connexin 26 (Cx26), is a member of the connexin family of gap junction proteins. GJB2 is overexpressed in various cancers, including triple-negative breast cancer (4), cervical cancer (5), hepatocellular carcinoma (6), and pancreatic cancer (7). It is closely linked to critical processes such as cell proliferation, apoptosis, migration, and radiosensitivity (8,9). Tang et al. identified elevated GJB2 expression in LUAD using The Cancer Genome Atlas (TCGA) database, suggesting its potential as a prognostic biomarker for poor survival in LUAD (10). Similarly, research by Li et al. observed significantly higher GJB2 expression in NSCLC cell lines compared to the human bronchial epithelial (HBE) cell line (11).
Cancer stem cells (CSCs) represent a small subset of cells within malignant tumors that possess self-renewal and differentiation capabilities (12), contributing to tumorigenesis, progression, recurrence, metastasis, and chemoresistance (13). Four transcription factors, sex-determining region Y-Box 2 (SOX2), octamer-binding transcription factor 4 (OCT4), cellular (c)-MYC, and Kruppel-like factor 4 (KLF4), enable the reprogramming of somatic cells into induced pluripotent stem cells, highlighting their importance in cancer biology (14). SOX2 has been recognized as a pivotal regulator of CSC properties, driving the self-renewal and tumor-initiating capacities of these cells, which contribute to tumor progression (15).
The nuclear factor kappa-B (NF-κB) signaling pathway is activated through two primary mechanisms: the canonical and non-canonical pathways. The canonical NF-κB pathway is frequently activated in various cancers and is closely linked to tumorigenesis and disease progression (16). This pathway is persistently active in CSCs across multiple cancer types, playing a crucial role in their proliferation, survival, and stemness maintenance of CSCs. Rinkenbaugh et al. demonstrated that NF-κB signaling is activated in glioblastoma stem cells (GSCs), with canonical and non-canonical pathways contributing to the GSC phenotype (17). Similarly, in lung CSCs, inhibition of NF-κB signaling using the specific inhibitor significantly reduces stemness, self-renewal, and migratory abilities (18).
Our study identified GJB2 as an essential gene related to CSC properties in LUAD. The expression of GJB2 was elevated in LUAD cell lines, which promoted cell proliferation and metastasis while inhibiting apoptosis. Sphere formation assays and flow cytometry confirmed that GJB2 enhanced the stem cell properties of LUAD cells. Mechanistically, GJB2 activated NF-κB signaling, leading to the nuclear translocation of p65 and the subsequent transcriptional upregulation of SOX2, thereby enhancing CSC properties. Our findings suggest that GJB2 plays a critical role in modulating CSC characteristics in LUAD through its regulation of the NF-κB/SOX2 axis. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2075/rc).
Methods
Bioinformatics analysis
According to the standard of P<0.05, use Gene Expression Omnibus (GEO) database to screen differentially expressed genes (DEGs). The Gene Expression Profiling Interactive Analysis (GEPIA), GEO, and University of ALabama at Birmingham CANcer (UALCAN) databases were used to evaluate the expression of GJB2 in LUAD tissues and normal lung tissues. The Kaplan-Meier database was used to evaluate the correlation between genes and the prognosis of lung cancer patients. GSEA was used to determine the relationship between GJB2 and related pathways and functions. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Cell lines and cell culture
The HBE cell lines and human LUAD cell lines A549, H1299, PC-9, H1975 were obtained from the Immocell (Xiamen, China). The cell line of H2126 cell line obtained from BDBIO (Hangzhou, China). HBE, H1299 and H2126 cell lines were routinely cultured in RPMI 1640 (Lonsera, China) with 10% fetal bovine serum BDBIO (Hangzhou, China). A549, PC-9 and H1975 were routinely cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS. All the cells were incubated at 37 ℃ in 5% CO2.
Western blotting
The total protein was extracted from variously treated cells using Laemmli 2× Concentrate (Sigam, S3401; USA). Separate nuclear protein and cytoplasmic protein according to the instructions of the kit (Beyotime, P0027; China). Protein per sample was separated by polyacrylamide gel electrophoresis and then transferred to nitrocellulose (NC) membrane (PALL, 66485; USA). The membranes were incubated with 5% non-fat milk for 1 h at room temperature and then probed with primary antibodies at 4 ℃ overnight and incubated with the corresponding HRP (Horseradish peroxidase)-conjugated secondary antibody. After incubation with a chemiluminescence kit (Tanon, 180-501; China), the protein bands were captured by a ChemiDoc Touch Imaging System (Bio-Rad, Hercules, USA). Anti-β-actin (42 kDa; AC004; RRID: AB_2737399), anti-Snail (29 kDa; A5243; RRID: AB_2766076) and anti-SOX2 (35 kDa; A0561; RRID: AB_2716820) antibodies were was purchased from ABclonal (Wuhan, China). Anti-IKB alpha (36 kDa; ET1603-6; RRID: AB_3065034), anti-Phospho-IKB alpha (S32) (36 kDa; ET1609-78; RRID: AB_3069887), anti-N-Cadherin (140 kDa; ET1607-37; RRID: AB_3069761), anti-E-Cadherin (135 kDa; ET1607-75; RRID: AB_3069782), anti-Vimentin (54 kDa; ET1610-39; RRID: AB_3069923), anti-GJB2 (26 kDa; ER1902-42; RRID: AB_3069426), and anti-OCT4 (45 kDa; EM100306; RRID: AB_3068737) antibodies were obtained from Huabio (Hangzhou, China), while Anti-KLF4 (50 kDa; WL02532) and anti-c-Myc (49 kDa; WL01781) antibodies from Wanleibio (Shenyang, China). Anti-p65 (65 kDa; 250060) and anti-p-p65 (65 kDa; 310013) were obtained from Zenbio (Chengdu, China).
Cell transfection
Cells were seeded in plates at 70–80% confluence. For plasmid, cells were transfected with PolyJet™ DNA transfection reagent (SignaGen, SL100688) according to the manufacturer’s instructions. The pCMV-SOX2(human)-3×FLAG (P57689), pGL3-Basic-SOX2(P21095), pCMV-GJB2-3×FLAG (P34905), pLV3-p65-3×FLAG (P62021) and pNF-kB-Luc (P0455) were obtained from MiaoLingBio (Wuhan, China). The pRL-CMV vector was purchased from Promega (Madison, WI, USA). The cells were transfected with siRNA mixed with GenMute (SignaGen, SL100568) when the cell density reached 40–50% confluence in a cell culture plate. Cells were cultured for 48 h before plating for protein or RNA isolation and various assays. GJB2 siRNA: 5'- CCCAGUUGUUAGAUUAAGAdTdT-3.
Cell counting kit-8 (CCK-8) assay
Cell proliferation was evaluated using CCK-8 (BL1055A, Biosharp, China). A total of 5,000 cells per well transfected with plasmid or siRNA were seeded in 96-well plates. Then the samples were incubated with CCK-8 reagent at 37 ℃ for 1 h after 24, 48, 72 and 96 h of incubation. According to manufacturer’s instruction, the cell proliferation rate was determined at 450 nm absorbance.
Clone formation assay
Cells were counted, plated in triplicate in 6-well plate at 1,000 cells transfected with siRNA per well and incubated for two weeks. The cells were then fixed with 4% paraformaldehyde for 15 min and stained with 0.1% crystal violet (G1059, Solarbio). After washing out the dye, we counted the number of clones and compared the results.
Flow cytometry
Cell apoptosis assay
After cell transfection, 1×106 cells were collected from each group. The cell apoptosis was detected using Annexin V-FITC/PI Apoptosis Detection Kit (BB-4101, Bestbio; China) according to the manufacturer’s instructions. Annexin V-FITC (5 µL) and PI (5 µL) were added to the 500 µL cell resuspension and incubated for 30 mins in the dark. The percentage of apoptotic cells were detected by a CytoFLEX flow cytometer (Beckman Coulter, CA, USA).
CD133+/CD44+ cells assay
Transfected cells (1×106) were harvested and resuspended in phosphate-buffered saline (PBS) for analysis. Cells were stained with anti-CD44-FITC-conjugated (11-0441-82) and anti-CD133-APC-conjugated (17-1338-42) antibodies (both from Invitrogen) for 30 minutes. The percentage of CD133+/CD44+ cells with CSC characteristics from LUAD cells were analyzed using flow cytometry.
Transwell assay
To evaluate cell migratory ability. Cells (3×104) of A549 and H1299 were inoculated into the upper chamber in 250 µL of serum-free medium and 500 µL of 10% serum-containing medium was added to the lower chamber. After another 48 h incubation, culture medium was discarded and the cells were fixed with 4% paraformaldehyde for 15 mins and stained with crystal violet for 30 mins. Cell migratory was quantified by visual counting after photographed by microscopy.
Sphere-forming assay
Inoculate A549 and H1299 cells at a density of 10,000 cells/well onto a low attachment 6-well plate (Corning, USA). Add 20 ng/mL epidermal growth factor (EGF, Sangon Biotech, China), 20 ng/mL Basic Fibroblast Growth Factor (Bfgf, Beyotime, China), 1% B-27 (Gibco, Rockville, USA), and 1x penicillin streptomycin solution (C0222, Beyotime, China) to serum-free DMEM/F12 (Gibco, Rockville, USA). After 14 d, cell spheres formed from the cells were counted.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was prepared from the various treated cells using Total RNA Extraction Kit (Promega, LS1040) according to the manufacturer’s instructions. Each RNA sample was then reverse transcribed into complementary DNA (cDNA) using GoScript™ Reverse Transcriptase (Promega, A2791). qRT-PCR was performed in triplicate using the GoTaq® qPCR and RT-qPCR (real time-quantitative polymerase chain reaction) Systems (Promega, A6001) with a Bio-Rad CXF96 PCR system (Hercules, CA, USA). 2−ΔΔCt means used to quantify the relative gene expression level, with β-actin as the reference gene. The primer sequences used for RT-qPCR are listed in Table 1.
Table 1
| Gene | Primer (5'>3') |
|---|---|
| CD133 | Forward: TTGGCTCAGACTGGTAAATCCC |
| Reverse: ATAGGAAGGACTCGTTGCTGGT | |
| CD90 | Forward: AAGCCAGGATTGGGGATGTG |
| Reverse: TGTGGCAGAGAAAGCTCCTG | |
| CD44 | Forward: CCCCATTACCAAAGACCACGA |
| Reverse: TTCTGCAGGTTCCGTGTCTC | |
| GJB2 | Forward: GCTGCAAGAACGTGTGCTAC |
| Reverse: TGGGTTTTGATCTCCTCGAT | |
| SOX2 | Forward: TACAGCATGTCCTACTCGCAG |
| Reverse: GAGGAAGAGGTAACCACAGGG | |
| β-actin | Forward: CCTTCCTGGGCATGGAGTCCT |
| Reverse: GGAGCAATGATCTTGATCTTC |
RT-qPCR, real time-quantitative polymerase chain reaction.
Dual luciferase reporter assays
The SOX2 promoter luciferase plasmid were co-transfected with Renilla luciferase vector into the cells. After 48 h, lyse the cells and use the Dual Luciferase Reporter Gene Assay Kit (11402ES60, YEASEN, China) to detect activities of Firefly Luciferase and Renilla Luciferase. All the results are presented as average value of triplicates ± standard deviation (SD).
Statistical analysis
Student’s t-test was used for comparing the variables between two groups, whereas, one-way analysis of variance (ANOVA) was used for the comparison of at least three groups. Statistical analysis was performed using GraphPad Prism 6 (La Jolla, CA, USA). The error bars in the figures represent mean ± SD from at least three independent experiments. P value <0.05 was considered statistically significant.
Results
Identification and screening of DEGs related to CSC properties in LUAD
DEGs from the GSE21612 dataset (which includes gene expression profiles from National Cancer Institute [NCI]-H1299 parental cells, CSCs, and xenograft tumors derived from both cell types) were initially analyzed to identify key molecules associated with CSC properties in LUAD. These findings were integrated with data from GSE136043 (comprising five fresh lung cancer tissues and five non-tumor tissues) and the National Center for Biotechnology Information-Gene (NCBI-Gene) database. This analysis led to the selection of candidate genes, including AHR, PDPN, GNAS, TFPI, LAMC2, GJB2, and DKK1 (Figure 1A). Subsequently, the Gene Expression Profiling Interactive Analysis 2 (GEPIA 2) database was utilized to validate the expression of these seven candidate genes in LUAD, revealing that only the differential expression of PDPN and GJB2 were significant (Figure 1B). Furthermore, the Kaplan-Meier database was employed to assess the correlation between the expression levels of PDPN and GJB2 and lung cancer prognosis. Our results indicated that only GJB2 expression was negatively associated with overall and first progress survival in patients (Figure 1C).
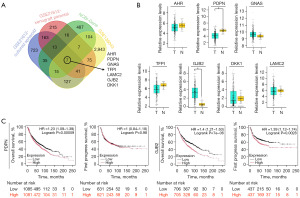
High expression of GJB2 is required for malignant behaviors in LUAD cells
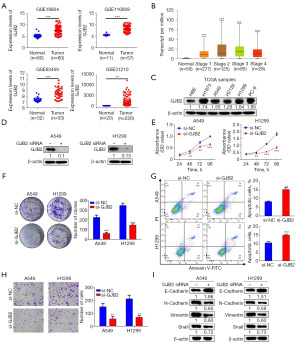
Analysis of four additional datasets from the GEO database demonstrated that GJB2 expression was substantially higher in lung cancer tissues compared to normal lung tissues (Figure 2A). According to the University of Alabama at Birmingham Cancer data analysis portal (UALCAN), GJB2 expression increases in stages 1 and 2 of LUAD; it gradually decreases in stages 3 and 4, remaining higher than normal tissues (Figure 2B). Consistently, GJB2 expression was significantly upregulated in human LUAD cell lines (H1975, A549, H2126, H1299, PC-9) compared to normal human bronchial epithelial cells (Figure 2C). The A549 and H1299 cell lines were selected for subsequent experiments. After 48 h of transfection with GJB2 small interfering RNA (siRNA), GJB2 expression decreased in both cell lines compared to the negative control (si-NC) group (Figure 2D). Cell Counting Kit-8 (CCK-8) assays demonstrated that silencing GJB2 significantly impaired the proliferative capabilities of A549 and H1299 cells (Figure 2E). GJB2 knockdown significantly inhibited colony formation in LUAD cells (Figure 2F). Flow cytometry analysis revealed that silencing GJB2 increased the percentage of apoptotic cells in the A549 and H1299 cell lines (Figure 2G). The effect of GJB2 on cell migration was examined using Transwell assays without Matrigel, demonstrating that LUAD cells transfected with GJB2 siRNA exhibited markedly decreased migratory capabilities (Figure 2H). Moreover, western blot analysis confirmed that GJB2 knockdown substantially reduced the expression of N-cadherin, vimentin, and Snail while increasing E-cadherin levels (Figure 2I). These results indicate that GJB2 influences the proliferation, apoptosis, and migration of LUAD cells.
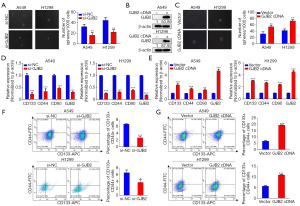
GJB2 promotes CSC properties in LUAD
The role of GJB2 in promoting lung CSC properties was further investigated. The sphere formation assay effectively assesses CSC properties. Tumor sphere cultures demonstrated that GJB2 knockdown considerably reduced the sphere size and the number of spheres formed in A549 and H1299 cells (Figure 3A). Conversely, western blot analysis indicated that GJB2 overexpression in these cell lines (Figure 3B) led to increased sphere formation size and efficiency (Figure 3C). The surface markers for lung CSCs have been identified as CD133 (19), CD44 (20), and CD90 (21). Strikingly, qRT-PCR analysis confirmed that GJB2 knockdown considerably reduced these gene expressions (Figure 3D), whereas GJB2 overexpression upregulated CD133, CD44, and CD90 (Figure 3E). Flow cytometry confirmed that the expression of CD133 and CD44 (22), which reflect CSC properties, was significantly regulated by GJB2 (Figure 3D,3E). Therefore, CD133+CD44+ cells were sorted for flow cytometric analysis. Results revealed that the ratio of CD133+/CD44+ cells was decreased in the GJB2 siRNA group (Figure 3F), while GJB2-overexpressing LUAD cells exhibited a significantly higher percentage of CD133+/CD44+ cells (Figure 3G). These results suggest that GJB2 promotes CSC properties in LUAD cells.
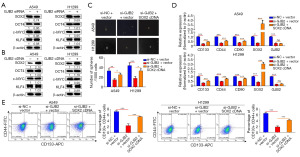
SOX2 mediates GJB2 to enhance CSC properties in LUAD cells
The mechanism by which GJB2 enhances lung CSC properties was investigated. The stem cell markers SOX2, OCT4, KLF4, and c-MYC are critical in inducing pluripotency in somatic cells and are employed to identify CSC subpopulations across various cancers (23). Initially, the expression of these CSC drivers (SOX2, OCT4, KLF4, and c-MYC) was assessed through western blotting. The results indicated that only SOX2 expression was downregulated following transfection with GJB2 siRNA, while GJB2 overexpression increased SOX2 levels (Figure 4A,4B). The knockdown efficiency of SOX2 was examined to confirm whether GJB2-mediated promotion of lung CSC properties relies on SOX2. Our findings demonstrated a substantial reduction in SOX2 expression. In A549 and H1299 cells, GJB2 siRNA and SOX2 complementary DNA (cDNA) co-transfection resulted in more spheroids than GJB2 siRNA transfection alone (Figure 4C). The qRT-PCR analysis revealed similar changes in the expression of CD133, CD44, and CD90 (Figure 4D). Flow cytometry results indicated that simultaneous overexpression of SOX2 increased the proportion of CD133+/CD44+cells compared to GJB2 knockdown alone (Figure 4E). These results suggest that GJB2 regulates CSC properties by enhancing SOX2 expression in LUAD cells.
GJB2 upregulates SOX2 expression by activating NF-κB activity in LUAD cells
Potential downstream pathways regulated by GJB2 were explored to elucidate further the mechanism by which GJB2 induces SOX2 expression. Gene set enrichment analysis (GSEA) revealed a close association between GJB2 and the NF-κB pathway (Figure 5A). The NF-κB signaling pathway includes the ubiquitination and proteasomal degradation of the inhibitor of nuclear factor kappa-B alpha (IκBα), leading to the translocation of p65 into the nucleus and activation of target genes. GJB2-mediated activation of the NF-κB signaling pathway was assessed in A549 and H1299 cells to determine whether the NF-κB pathway contributes to the GJB2-mediated regulation of SOX2. The GJB2 knockdown inhibited the phosphorylation of IκBα and p65, thereby suppressing the NF-κB pathway (Figure 5B). Overexpression of GJB2 resulted in increased phosphorylation of IκBα and p65, activating the NF-κB pathway (Figure 5C). Dual luciferase reporter gene assays were conducted to investigate this relationship further, which confirmed that GJB2 regulates NF-κB pathway activity (Figure 5D,5E). Given the significance of p65 nuclear translocation for NF-κB transcriptional activity (24), the effect of GJB2 on the translocation of p65 was investigated. Knockdown of GJB2 caused p65 accumulation in the cytoplasm and a corresponding reduction in the nucleus (Figure 5F). Conversely, overexpression of GJB2 facilitated the translocation of p65 from the cytoplasm to the nucleus (Figure 5G). SOX2 levels were analyzed following p65 cDNA overexpression using western blotting to determine whether NF-κB regulates SOX2 expression. In cells transfected with GJB2 siRNA and p65 cDNA, SOX2 expression was notably elevated compared to those transfected with GJB2 siRNA alone (Figure 5H). Furthermore, treatment with NF-κB pathway inhibitors revealed that overexpression of GJB2 significantly increased SOX2 levels, which were subsequently inhibited by NF-κB-IN-1 (Figure 5I).
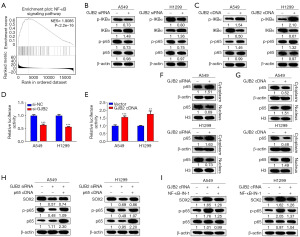
GJB2 promotes SOX2 transcription through NF-κB pathway activation
As it is well known, activation of the NF-κB pathway triggers nuclear translocation of p65 and transcription of target genes. It was necessary to clarify whether GJB2-induced SOX2 expression results from NF-κB pathway activation. SOX2 messenger RNA (mRNA) levels were assessed following GJB2 knockdown or overexpression. The qRT-PCR analysis revealed that SOX2 mRNA levels decreased upon GJB2 knockdown (Figure 6A) and increased following GJB2 overexpression (Figure 6B). Moreover, a luciferase reporter plasmid carrying the SOX2 promoter was utilized to detect changes in promoter activity. Strikingly, GJB2 silencing diminished SOX2 promoter activity in both A549 and H1299 cells (Figure 6C). Conversely, SOX2 promoter activity was significantly enhanced by GJB2 cDNA transfection in LUAD cells (Figure 6D), indicating that GJB2 influences SOX2 expression at the transcriptional level. Furthermore, qRT-PCR confirmed that either p65 overexpression or NF-κB inhibitors such as NF-κB-IN-1 could regulate SOX2 mRNA expression (Figure 6E,6F). Consistent findings were observed in luciferase reporter assays, affirming the regulatory role of GJB2 in SOX2 transcription (Figure 6G,6H). Our data suggest that GJB2 promotes SOX2 transcription, with the NF-κB pathway playing a critical role in this process.
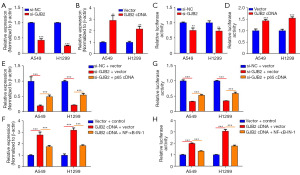
Discussion
LUAD represents the most prevalent histological subtype of lung cancer (25). This study utilized GEO data to determine the correlation between GJB2 expression and the stem cell characteristics of LUAD. The GEPIA 2 and GEO databases corroborated the significant upregulation of GJB2 in LUAD. Our experimental findings indicate that GJB2 is highly expressed in LUAD cell lines, underscoring its critical role in promoting the malignant phenotype of these cells. GJB2 silencing inhibited LUAD cell proliferation and migration in vitro while promoting apoptosis. GJB2 enhances the CSC properties of LUAD cells. A novel mechanism is proposed in which GJB2 activates the NF-κB pathway, facilitating the nuclear translocation of p65, which leads to upregulation of SOX2 expression. This series of results provides new mechanistic insights into GJB2 regulatory role in CSC properties in LUAD.
A systematic pan-cancer analysis revealed that GJB2 is highly expressed across various tumors, significantly influencing cancer development and tumor immunity (26). GJB2 is elevated in HCC cells, where its high expression correlates with poor prognosis. GJB2 promotes HCC progression by activating glycolysis through cytoplasmic translocation, contributing to an inhibitory tumor microenvironment. Furthermore, research has indicated that the positive regulatory feedback between GJB2 and the PI3K/Akt signaling pathway fosters epithelial-mesenchymal transition via gap junction intercellular communication (GJIC)-independent pathways, resulting in gefitinib resistance in NSCLC (6). However, findings regarding GJB2 expression in LUAD have been inconsistent, with some studies reporting high levels (10,27,28) while others indicate low expression (29,30). This discrepancy may stem from differing interpretations of GJB2 promoter methylation levels. Our study reinforces that GJB2 is highly expressed in LUAD and is closely associated with the proliferation, metastasis, and apoptosis of LUAD.
CSCs are recognized as pivotal contributors to tumor initiation, progression, and recurrence (31). Thiagarajan et al. demonstrated that GJB2 is upregulated in the CSCs of triple-negative breast cancer, where it promotes CSC self-renewal by forming signaling complexes with multipotent transcription factors such as Nanog and FAK, which stabilize Nanog and activate FAK (4). Similarly, the research by Zekri et al. confirmed the high expression of GJB2 in breast CSCs, further suggesting a strong correlation between GJB2 and tumor stem cell properties (32). Our results indicate that GJB2 promotes CSC properties in LUAD. SOX2, a critical reprogramming factor, facilitates the conversion of non-CSCs into CSCs, thereby supporting tumorigenesis and self-renewal (33). Our results indicate that SOX2 is essential for GJB2’s promotion of lung CSC properties, as GJB2 upregulates SOX2 expression. Silencing SOX2 reverses the increase in the number and size of cell spheroids and the population of CD133+/CD44+cells induced by GJB2, indicating that SOX2 mediates GJB2 regulatory effects of CSC characteristics in LUAD.
The NF-κB pathway is well-established for its crucial role in regulating immune responses and inflammation. In addition, its significant involvement in tumorigenesis is increasingly recognized. This pathway is frequently aberrantly or constitutively activated in many malignant tumors (34). It is persistently active in CSCs across different cancers, playing a vital role in their maintenance (35-37). This study demonstrated that GJB2 activates the NF-κB pathway, promoting the nuclear translocation of p65 and enhancing SOX2 transcription. Although the mechanism by which GJB2 activates NF-κB has not been elucidated, Liu’s research suggests that GJB2 may induce the ubiquitin-mediated degradation of IκBα, thereby activating the NF-κB pathway. Our findings provide a new insight into how GJB2 regulates SOX2 expression.
Conclusions
We described a novel mechanism in which GJB2 promotes the transcription of SOX2 by activating the NF-κB pathway. The results of this study provide possible molecular targets for the treatment of LUAD. However, the specific potential mechanism by which GJB2 activates the NF-κB pathway in LUAD deserves further investigation.
Acknowledgments
We are very grateful for the support of the 289 Project of Anhui Provincial Healthcare Commission. We would like to thank EditChecks (https://editchecks.com.cn/) for providing linguistic assistance during the preparation of this manuscript.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2075/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2075/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2075/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2075/coif). S.Z. reports receiving funding support from the Natural Science Foundation of Anhui Education Department (No. 2023AH040264), the Anhui Provincial Health Research Project (No. AHWJ2023BAc10056) and the Health Development Promotion Project-Spark Program (No. XHJH-0058). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Testa U, Castelli G, Pelosi E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers (Basel) 2018;10:248. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Thiagarajan PS, Sinyuk M, Turaga SM, et al. Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nat Commun 2018;9:578. [Crossref] [PubMed]
- Meng S, Liu Y, Wang X, et al. The prognostic value and biological significance of gap junction beta protein 2 (GJB2 or Cx26) in cervical cancer. Front Oncol 2022;12:907960. [Crossref] [PubMed]
- Liu H, Li X, Zhang C, et al. GJB2 Promotes HCC Progression by Activating Glycolysis Through Cytoplasmic Translocation and Generating a Suppressive Tumor Microenvironment Based on Single Cell RNA Sequencing. Adv Sci (Weinh) 2024;11:e2402115. [Crossref] [PubMed]
- Zhu T, Gao YF, Chen YX, et al. Genome-scale analysis identifies GJB2 and ERO1LB as prognosis markers in patients with pancreatic cancer. Oncotarget 2017;8:21281-9. [Crossref] [PubMed]
- Shettar A, Damineni S, Mukherjee G, et al. Gap junction β 2 expression is negatively associated with the estrogen receptor status in breast cancer tissues and is a regulator of breast tumorigenesis. Oncol Rep 2018;40:3645-53. [Crossref] [PubMed]
- Li Y, Yang L, Tao R, et al. The Expression of Connexin 26 Regulates the Radiosensitivity of Hepatocellular Carcinoma Cells through a Mitogen-Activated Protein Kinases Signal Pathway. Int J Mol Sci 2022;23:14644. [Crossref] [PubMed]
- Tang Y, Zhang YJ, Wu ZH. High GJB2 mRNA expression and its prognostic significance in lung adenocarcinoma: a study based on the TCGA database. Medicine (Baltimore) 2020;99:e19054. [Crossref] [PubMed]
- Li S, Liu Y, Qiu G, et al. Long Non-Coding RNA CAR10 Facilitates Non-Small Cell Lung Cancer Cell Migration and Invasion by Modulating the miR-892a/GJB2 Pathway. Cancer Manag Res 2021;13:1967-79. [Crossref] [PubMed]
- Nassar D, Blanpain C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu Rev Pathol 2016;11:47-76. [Crossref] [PubMed]
- Zeng Z, Fu M, Hu Y, et al. Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer. Mol Cancer 2023;22:172. [Crossref] [PubMed]
- Huang T, You Q, Huang D, et al. A positive feedback between PDIA3P1 and OCT4 promotes the cancer stem cell properties of esophageal squamous cell carcinoma. Cell Commun Signal 2024;22:60. [Crossref] [PubMed]
- Zhu Y, Huang S, Chen S, et al. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis 2021;12:449. [Crossref] [PubMed]
- Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem 2010;336:25-37. [Crossref] [PubMed]
- Rinkenbaugh AL, Cogswell PC, Calamini B, et al. IKK/NF-κB signaling contributes to glioblastoma stem cell maintenance. Oncotarget 2016;7:69173-87. [Crossref] [PubMed]
- Zakaria N, Mohd Yusoff N, Zakaria Z, et al. Inhibition of NF-κB Signaling Reduces the Stemness Characteristics of Lung Cancer Stem Cells. Front Oncol 2018;8:166. [Crossref] [PubMed]
- Huang T, Zhou X, Mao X, et al. Lactate-fueled oxidative metabolism drives DNA methyltransferase 1-mediated transcriptional co-activator with PDZ binding domain protein activation. Cancer Sci 2020;111:186-99. [Crossref] [PubMed]
- Leung EL, Fiscus RR, Tung JW, et al. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One 2010;5:e14062. [Crossref] [PubMed]
- Yan X, Luo H, Zhou X, et al. Identification of CD90 as a marker for lung cancer stem cells in A549 and H446 cell lines. Oncol Rep 2013;30:2733-40. [Crossref] [PubMed]
- Hao S, Li F, Jiang P, et al. Effect of chronic intermittent hypoxia-induced HIF-1α/ATAD2 expression on lung cancer stemness. Cell Mol Biol Lett 2022;27:44. [Crossref] [PubMed]
- van Schaijik B, Davis PF, Wickremesekera AC, et al. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: a review. J Clin Pathol 2018;71:88-91. [Crossref] [PubMed]
- Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys 2013;42:443-68. [Crossref] [PubMed]
- Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012;150:1107-20. [Crossref] [PubMed]
- Jia Y, Guo B, Zhang W, et al. Pan-cancer analysis of the prognostic and immunological role of GJB2: a potential target for survival and immunotherapy. Front Oncol 2023;13:1110207. [Crossref] [PubMed]
- Yang J, Qin G, Luo M, et al. Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC-independent induction of EMT. Cell Death Dis 2015;6:e1829. [Crossref] [PubMed]
- Lu A, Shi Y, Liu Y, et al. Integrative analyses identified ion channel genes GJB2 and SCNN1B as prognostic biomarkers and therapeutic targets for lung adenocarcinoma. Lung Cancer 2021;158:29-39. [Crossref] [PubMed]
- Li DY, Yue LX, Wang SG, et al. Quercitrin restrains the growth and invasion of lung adenocarcinoma cells by regulating gap junction protein beta 2. Bioengineered 2022;13:6126-35. [Crossref] [PubMed]
- Chen Y, Hühn D, Knösel T, et al. Downregulation of connexin 26 in human lung cancer is related to promoter methylation. Int J Cancer 2005;113:14-21. [Crossref] [PubMed]
- Raniszewska A, Kwiecień I, Rutkowska E, et al. Lung Cancer Stem Cells-Origin, Diagnostic Techniques and Perspective for Therapies. Cancers (Basel) 2021;13:2996. [Crossref] [PubMed]
- Zekri AN, Bahnassy A, Mourad M, et al. Genetic profiling of different phenotypic subsets of breast cancer stem cells (BCSCs) in breast cancer patients. Cancer Cell Int 2022;22:423. [Crossref] [PubMed]
- Boumahdi S, Driessens G, Lapouge G, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 2014;511:246-50. [Crossref] [PubMed]
- Dolcet X, Llobet D, Pallares J, et al. NF-kB in development and progression of human cancer. Virchows Arch 2005;446:475-82. [Crossref] [PubMed]
- Rajasekhar VK, Studer L, Gerald W, et al. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-κB signalling. Nat Commun 2011;2:162. [Crossref] [PubMed]
- Garner JM, Fan M, Yang CH, et al. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor κB signaling in glioblastoma cancer stem cells regulates the Notch pathway. J Biol Chem 2013;288:26167-76. [Crossref] [PubMed]
- Sun L, Mathews LA, Cabarcas SM, et al. Epigenetic regulation of SOX9 by the NF-κB signaling pathway in pancreatic cancer stem cells. Stem Cells 2013;31:1454-66. [Crossref] [PubMed]


