
Extracellular vesicles isolated from SphK1 inhibitor SKI II-medium restrain the migration of colorectal cancer
Highlight box
Key findings
• Extracellular vesicles (EVs) extracted from RKO exosom-free serum culture medium-mediated colorectal cancer cell line RKO and HT29 cell migration were partially reversed by EVs which extracted from RKO exosom-free serum culture medium with SKI II, an inhibitor of SphK1, suggesting that SKI II may play a therapeutic role in EV-mediated colorectal cancer cell migration.
What is known and what is new?
• Sphingosine kinase 1 (SphK1) is known to promote the transfer of several tumors. SKI II is an inhibitor of SphK1.
• This study shows that EVs isolated from SphK1 inhibitor SKI II-medium increased E-cadherin and decreased vimentin in colorectal cancer cells and inhibited cell migration.
What is the implication, and what should change now?
• SphK1 inhibitor encapsulated within EVs may emerge as a form of treatment in the future. Selecting appropriate EVs, standardizing engineered EVs, and developing reliable methods for EV extraction and quantification are the main challenges for clinical translation.
Introduction
Colorectal cancer is a chronic disease particularly detrimental to human health. The incidence of colorectal cancer has escalated among patients below the age of 50 years. The prognosis for colorectal cancer patients with unresectable metastases remains suboptimal. Thus, new therapeutic strategies remain urgently needed (1).
Extracellular vesicles (EVs) are endogenous membrane-like particles secreted by cells, formed through invagination of the plasma membrane, and transport cell surface proteins, soluble proteins, and small molecules such as lipids, nucleic acids, and proteins. Recently, EVs have become important mediators of intercellular communication. It is important for tumor growth and metastasis by promoting metastasis, angiogenesis, immune regulation and tissue regeneration (2,3). Interestingly, EVs were found to be responsible for sphingosine kinase 1 (SphK1) transport and release into the tumor microenvironment to promote ovarian tumor progression (4). Study has shown that when the encapsulated “cargo” is taken up by recipient cells via exosome uptake, the “cargo” will be involved in regulating various metabolic processes within cancer cells (5). Thus, exosomes are considered potential drug carriers because of their ability to safely deliver biomolecules to recipient cells (6-8). In the present study, we attempted to extract EVs from exosomes-free serum cultures of human colon adenocarcinoma cells (RKO) treated with the SphK1 inhibitor SKI II, and the EVs were used to interfere with RKO cells and HT29 cells to investigate the effects of exosomes isolated from the culture medium of SphK1 inhibitor SKI II on the expression of E-cadherin and vimentin and cell migration of colorectal cancer cells. Our study will help to expand the application of EVs in the biomedical field.
SphK1 is proven to promote the transfer of several tumors. Sphingolipid 1-phosphate (S1P) produced by SphK1 could be exported outside the cell through specific transporters to trigger oncogenic sphingolipid signaling (9). Study has shown that SphK1 was overexpressed in colorectal cancer tissues and is associated with enhanced metastasis (10). Our previous study also showed that SphK1 increased vimentin expression, decreased E-cadherin expression, and promoted colorectal cancer cell migration (11). Although there have been many studies on colorectal cancer and EVs, it is unknown whether SKI II, an inhibitor of SphK1, uses EVs as transporters to inhibit colorectal cancer migration. The aim of this study was to investigate the effects of EVs isolated from the culture medium of SphK1 inhibitor SK I II on E-cadherin and vimentin expression and the migration ability of colorectal cancer cells. Our results first showed that EVs isolated from SphK1 inhibitor SKI II-medium affect E-cadherin and vimentin expression in colorectal cancer cells and inhibit cell migration. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2152/rc).
Methods
Cell culture and cell viability assays
Colorectal cancer cell lines HT29 (CL-0118, Procell, China) and RKO (CX0083, BOSTER, China) were cultured at 37 ℃ in 5% CO2. EVs inhibitor (GW4869) was purchased from Umibio, China (Lot: 45089153). SKI II, an inhibitor of SphK1, was purchased from Selleck, China (S717602). Cell viability was analyzed by a cell counting kit-8 (CCK-8) (GK10001, GLPBIO, USA). Cells were seeded at equivalent densities in a 96-well plate. When the cells fully recovered their morphology, varying concentrations of SKI II (0, 2.5, 5, 10 µM) were added, respectively. After 48 hours, the culture media was removed from the cells and 10 µL of CCK-8 solution and 90 µL Dulbecco’s modified Eagle medium (DMEM) were added. The plate was incubated for 1 hour at 37 ℃. Next, each well was measured by a microplate reader (Synergy H1, USA) at 450 nm.
Isolation, characterization and quantification of EVs
Cells were cultured for 48 hours in 0 and 5 µM of SKI II-containing 10% exosome-depleted-foetal bovine serum (FBS) complete medium. The media was collected. Exosome Extraction and Purification Kit was purchased from Umibio, China (UR52121). The media was processed using the Kit to eliminate cell debris and precipitate, re-suspended, and purified EVs. The EVs were stored at −80 ℃. Nanoparticle tracking analysis (NTA, Particle Metrix, German), a transmission electron microscope (TEM, HITACHI HT-7700, Japan), and western blotting were used to identify EVs. In addition, the obtained EVs were quantified by the bicinchoninic acid (BCA) method.
EVs labeling and uptake
PKH67 (UR52303) was obtained from Umibio, China. PKH67 was applied to label EVs with green fluorescence. After 24 hours of co-culture, 4% paraformaldehyde was used to fix the cells, and then the Antifade mounting medium with 4’,6-diamidino-2-phenylindole (DAPI) was used. DAPI (P0131) was obtained from Beyotime, China. The uptake of EVs for the cell was observed by fluorescence microscopy.
Western blotting analysis
A special purpose lysate was used to extract the total protein from EVs. The special purpose lysate (UR33101) was got from Umibio, China. Total protein from cells was extracted with radio-immunoprecipitation assay (RIPA) buffer. sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate protein samples. Then the protein samples were transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were obtained from Millipore, USA. 5% milk blocked the membranes for 2 hours at room temperature. The primary antibody was incubated on the membrane overnight at 4 ℃. The next day, secondary antibody was incubated on the membrane for 1 hour at room temperature. An enhanced chemiluminescence gel imaging system, obtained from Bio-Rad, detected the bands. Lastly, Image J software was used to analyze the blots. Besides, CD81 (66866-1-lg, 1:1,000), TSG101 (28283-1-AP, 1:4,000), E-cadherin (60335-1-lg, 1:4,000), Vimentin (10366-1-AP, 1:2,000) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (10494-1-AP, 1:4,000) were obtained from Proteintech®, USA. Calnexin (HY-P80578, 1:1,000) was obtained from MedChemExpress, China. Anti-mouse lgG (H+L) (BA1050; 1:10,000) and anti-rabbit lgG (H+L) (BA1054; 1:10,000) were obtained from BOSTER, China.
Transwell assay
Colorectal cancer suspension cultures were established using a 1% FBS medium. 200 µL of colorectal cancer cell suspension was added to the Transwell (Corning, USA) upper chamber. The lower chamber was added with 500 µL medium including 30% FBS. 24 hours later, the cells were fixed with 4% paraformaldehyde for 20 minutes. Then the cells were stained with 0.1% crystal violet for 8 minutes. A microscope was used to count the cells.
Statistical analysis
SPSS 26.0 and GraphPad Prism 8.0 software were used for statistical analyses. Statistically significant differences were calculated using a Student’s t-test and analysis of variance (ANOVA), followed by least significant difference (LSD)’s test. In western blotting analysis, GAPDH was used as a control, and the control group was homogenized.
Results
Identification of colorectal cancer cell-derived EVs
EVs were isolated from 10% exosome-depleted-FBS complete media derived from RKO cell lines, named as RKOEVs. The EVs obtained were identified by TEM, NTA and western blotting (12,13). As shown in Figure 1, upon TEM (the first image from left to right), the morphology of EV was shown to be of a typical spheroidal form with a saucer-like structure. The isolated EVs were also analyzed by NTA (the second image from left to right), which confirmed RKOEVs to have a mean size of 128.3±55.5 nm. Western blotting (the third image from left to right) showed that the typical EV markers CD81 and TSG101 was presented, but a deficiency of calnexin.

RKOEVs play a key role in E-cadherin and vimentin expression and migration of RKO cells
Colorectal cancer cells were cultured in 10% exosome-depleted FBS complete medium. To investigate whether RKOEVs played a key role in E-cadherin and vimentin expression and migration of colorectal cancer, RKO cells were treated for 48 hours with either EVs or 20 µM GW4869, an EV inhibitor. As shown in Figure 2A, the cells treated with GW4869 displayed markedly reduced expression of vimentin but increased E-cadherin when compared to the control group. Moreover, cell migration diminished when cells were treated with GW4869. On the other hand, RKO cells were co-cultured with PKH67-labeled RKOEVs. In Figure 2B, the fluorescently labelled RKOEVs could be observed in RKO cells. The RKO cells treated with RKOEVs showed decreased E-cadherin expression, increased vimentin expression and cell migration number, compared with the control ones (Figure 2C).

RKOEVs-mediated RKO cell migration was partially reversed by RKO-SKEVs
SKI II, an analog of sphingosine, inhibits SphK1 in a concentration-time dependent manner (14). RKO cells were incubated in media containing differing concentrations of SKI II for 48 hours, and the cell proliferation was determined using a CCK-8 cell viability assay. As shown in Figure 2D, of the concentrations (2.5, 5 and 10 µM) tested, the cell survival rate was 55.71% when the cells were tested with 5 µM SKI II. It suggested that among these 5 µM was the maximum concentration of SKI II tolerated by the cells. Cells were cultured for 48 hours in 5 µM of SKI II-containing 10% exosome-depleted-FBS complete medium, and EVs (RKO-SKEVs) were collected from the media of RKO cells. To investigate the impact on expression of E-cadherin and vimentin and migration of colorectal cancer when cancer cells uptake EVs isolated from SKI II-medium, RKO cells were co-cultured with 100 µg/mL of RKOEVs or RKO-SKEVs for 48 hours. The difference between the groups at that time was the component within EVs. Compared to cells treated with RKOEVs (RKO-RKOEVs group), the RKO cells treated with RKO-SKEVs (RKO-RKO-SKEVs group) demonstrated markedly reduced expression of vimentin, increased E-cadherin expression, and lower cell migration (Figure 2C). The data demonstrate that EVs isolated from SKI II-medium partially suppressed the migration induced by RKOEVs in colorectal cancer.
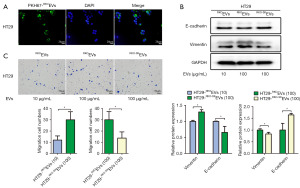
RKOEVs promoted HT29 cell migration while RKOEVs-mediated HT29 cell migration was partially reversed by RKO-SKEVs
HT29 colorectal cancer cells have weak metastatic potential, whilst the RKO cell line is a highly metastatic (15,16). Based on the diversity of malignant tumor differentiation, to explore the effect of EVs secreted by highly metastatic cancer cells on cells with low metastatic potential, we examined the effects of different amounts of RKOEV (10 µg/mL, 100 µg/mL) and RKO-SKEVs on E-cadherin and vimentin expression and cell migration in HT29 cells. Firstly, PKH67-labeled RKOEVs were co-cultured with HT29 cells. Fluorescently labelled RKOEVs were detected in HT29 cells (Figure 3A, the PBS group in Figure 2B was also its control group), suggesting that HT29 cells are able to uptake RKOEVs. HT29 cells were then treated with 10 µg/mL RKOEVs, 100 µg/mL RKOEVs, and 100 µg/mL RKO-SKEVs for 48 hours. When compared to cells treated with a lower concentration of EVs (10 µg/mL RKOEVs), HT29 cells treated with 100 µg/mL RKOEVs showed markedly increased vimentin expression, whilst E-cadherin was reduced (Figure 3B). Furthermore, cell migration was significantly enhanced (Figure 3C). This suggested that RKOEVs enhanced the migration of HT29 cells. Additionally, when compared to cells treated with RKOEVs, HT29 cells treated with RKO-SKEVs, displayed reduced vimentin levels and increased E-cadherin expression (Figure 3B). Cell migration was also weakened (Figure 3C). These data demonstrate that RKO-SKEVs partly restrain migration induced by RKOEVs in HT29 cells.
Discussion
Fifteen to twenty-five percent of patients develop metastasis after radical resection of the primary colorectal cancer, and one of the main reasons is that the tumor microenvironment does not change significantly after surgery. Tumor-derived EVs are important members of the tumor microenvironment. In recent years, the application potential of EV engineering in tumor therapy has paid much attention. The EV structure allows the use of various methods to modify its original configuration, including genetic engineering, chemical procedures, physical techniques, and microfluidic technologies, to load EV additional “cargo” for expanding its applications in the biomedical field. It is of great significance to further study the metastasis mechanism of colorectal cancer and find new treatment methods for colorectal cancer to prolong the survival of patients with advanced colorectal cancer.
EVs are key players in tumor metastasis (17). EVs could metastasize from primary tumor cells to distant sites, triggering epithelial-mesenchymal transition (EMT) and establishing a favorable premetastatic microenvironment for cancer cells’ later colonization (18,19). EMT is accompanied by decreased E-cadherin and increased vimentin. EVs secreted by colorectal cancer cells with EMT characteristics induce the polarization of M2 macrophages, which helps to form an immunosuppressive tumor microenvironment (20), and induce hepatocytes to remodel the liver microenvironment to form a “soil” supporting cancer cell colonization (21). These studies indicate that EVs derived from colorectal cancer cells play a key role in cancer cell metastasis. Complementarily, our study showed that colorectal cancer cells were able to ingest EVs secreted by cognate cells and regulate E-cadherin and vimentin expression to enhance cancer cell migration. Novel, we found that RKOEVs promoted not only RKO cell migration but also HT29 cell migration. It perhaps suggested that the reason of colorectal cancer with a small number of poorly differentiated cells has a worse prognosis than colorectal cancer with a single well-differentiated cell may be not only attributed to the invasion of poorly differentiated cells, but also due to the EVs released by high metastatic potential cancer cells being acquired by low metastatic potential cells, promoting the development of low metastatic potential cancer cells to higher metastatic potential.
In addition, due to the characteristics of low immune clearance, good tolerance, few side effects, and the ability to effectively deliver functional substances to diseased cells, the potential applicability of exosomes as carriers for cancer therapy has become increasingly prominent (22,23). Evidence suggested that changing composition of EVs, for instance, programmed cell death protein 1 (PD-1) and non-coding RNA, contributed to the tumor microenvironment remodeling (24). But the selection of cargo, appropriate dosages, the EV cargo loading methods and the optimal therapeutic modalities for cancer treatment remain elusive. EVs with therapeutic effects are a new strategy for the treatment of colorectal cancer in the future. SphK1 is overexpressed in a variety of cancers, promoting tumor cell proliferation (25), inducing EMT (26), helping cancer cells acquire stem-like properties (27), regulating tumor immune function (28), and finally promoting cancer cell metastasis (29). It has been reported that EVs secreted by ovarian cancer transfer SphK1 to the tumor microenvironment to promote the progression of ovarian cancer (4). In this study, we collected RKO cell culture medium cultured for 48 hours with 10% exosome-free serum complete medium supplemented with SphK1 inhibitor SKI II intervention, and then the EVs were extracted. The EVs were used to interfere with RKO and HT29 cells. The results showed that RKOEVs-mediated RKO and HT29 cell migration was partially reversed by RKO-SKEVs, suggesting that SKI II may play a therapeutic role in EV-mediated colorectal cancer cell migration.
In summary, we deduce that colorectal cancer-derived EVs not only increased vimentin expression and decreased E-cadherin expression in homologous cancer cells, but also had the same effect on the cancer cells with lower metastatic potential, and jointly promoted colorectal cancer metastasis. EVs isolated from SKI II medium were able to reverse cancer cell metastasis mediated by tumor-derived EVs.
In the future, a better understanding of the functional and compositional changes of EVs obtained from SphK1 inhibitor culture medium will provide more beneficial help for the development of new genetically engineered EVs for inhibiting colorectal cancer metastasis. Currently, human mesenchymal stroma/stem-like cells derived paclitaxel-loaded EVs have been revealed to have significant cytotoxic effects in carcinomas of lung, breast, ovary, colon, and astrocytoma cell lines in a concentration-dependent manner (30). Regrettably, there are no recognized engineered EVs that could be used for cancer therapy at present. Selecting appropriate EVs is the main challenge for clinical translation.
Conclusions
EVs isolated from SphK1 inhibitor SKI II-medium affect E-cadherin and vimentin expression in colorectal cancer and inhibit cell migration.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2152/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2152/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2152/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2152/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fan S, Zhou L, Zhang W, et al. Ferroptosis: the balance between death and survival in colorectal cancer. Int J Biol Sci 2024;20:3773-83. [Crossref] [PubMed]
- Zhou X, Jia Y, Mao C, et al. Small extracellular vesicles: Non-negligible vesicles in tumor progression, diagnosis, and therapy. Cancer Lett 2024;580:216481. [Crossref] [PubMed]
- Zhou X, Yan Y, Shen Y, et al. Exosomes: Emerging Insights into the Progression of Pancreatic Cancer. Int J Biol Sci 2024;20:4098-113. [Crossref] [PubMed]
- Gupta P, Kadamberi IP, Mittal S, et al. Tumor Derived Extracellular Vesicles Drive T Cell Exhaustion in Tumor Microenvironment through Sphingosine Mediated Signaling and Impacting Immunotherapy Outcomes in Ovarian Cancer. Adv Sci (Weinh) 2022;9:e2104452. [Crossref] [PubMed]
- Dixson AC, Dawson TR, Di Vizio D, et al. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat Rev Mol Cell Biol 2023;24:454-76. [Crossref] [PubMed]
- Wang Z, Popowski KD, Zhu D, et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat Biomed Eng 2022;6:791-805. [Crossref] [PubMed]
- Popowski KD, Moatti A, Scull G, et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter 2022;5:2960-74. [Crossref] [PubMed]
- Xiao Y, Wu M, Xue C, et al. Recent Advances in the Development of Membrane-derived Vesicles for Cancer Immunotherapy. Curr Drug Deliv 2024;21:403-20. [Crossref] [PubMed]
- Hii LW, Chung FF, Mai CW, et al. Sphingosine Kinase 1 Signaling in Breast Cancer: A Potential Target to Tackle Breast Cancer Stem Cells. Front Mol Biosci 2021;8:748470. [Crossref] [PubMed]
- Long J, Xie Y, Yin J, et al. SphK1 promotes tumor cell migration and invasion in colorectal cancer. Tumour Biol 2016;37:6831-6. [Crossref] [PubMed]
- Liu SQ, Xu CY, Wu WH, et al. Sphingosine kinase 1 promotes the metastasis of colorectal cancer by inducing the epithelial-mesenchymal transition mediated by the FAK/AKT/MMPs axis. Int J Oncol 2019;54:41-52. [PubMed]
- Sierra-López F, Castelan-Ramírez I, Hernández-Martínez D, et al. Extracellular Vesicles Secreted by Acanthamoeba culbertsoni Have COX and Proteolytic Activity and Induce Hemolysis. Microorganisms 2023;11:2762. [Crossref] [PubMed]
- Lin A, He W. LINC01705 derived from adipocyte exosomes regulates hepatocyte lipid accumulation via an miR-552-3p/LXR axis. J Diabetes Investig 2023;14:1160-71. [Crossref] [PubMed]
- French KJ, Schrecengost RS, Lee BD, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res 2003;63:5962-9. [PubMed]
- Maltseva DV, Makarova JA, Khristichenko AY, et al. Epithelial to Mesenchymal Transition Marker in 2D and 3D Colon Cancer Cell Cultures in the Presence of Laminin 332 and 411. Mol Biol (Mosk) 2019;53:330-8. [PubMed]
- Pan S, Deng Y, Fu J, et al. Decreased expression of ARHGAP15 promotes the development of colorectal cancer through PTEN/AKT/FOXO1 axis. Cell Death Dis 2018;9:673. [Crossref] [PubMed]
- Shen Y. TanTai J. Exosomes secreted by metastatic cancer cells promotes epithelial mesenchymal transition in small cell lung carcinoma: The key role of Src/TGF-β1 axis. Gene 2024;892:147873. Erratum in: Gene 2025;933:148934. [Crossref] [PubMed]
- Hussen BM, Abdullah ST, Abdullah SR, et al. Exosomal non-coding RNAs: Blueprint in colorectal cancer metastasis and therapeutic targets. Noncoding RNA Res 2023;8:615-32. [Crossref] [PubMed]
- Dokhanchi M, Pakravan K, Zareian S, et al. Colorectal cancer cell-derived extracellular vesicles transfer miR-221-3p to promote endothelial cell angiogenesis via targeting suppressor of cytokine signaling 3. Life Sci 2021;285:119937. [Crossref] [PubMed]
- Yang C, Dou R, Wei C, et al. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol Ther 2021;29:2088-107. [Crossref] [PubMed]
- Pucci M, Moschetti M, Urzì O, et al. Colorectal cancer-derived small extracellular vesicles induce TGFβ1-mediated epithelial to mesenchymal transition of hepatocytes. Cancer Cell Int 2023;23:77. [Crossref] [PubMed]
- Zuppone S, Zarovni N, Vago R. The cell type dependent sorting of CD9- and CD81 to extracellular vesicles can be exploited to convey tumor sensitive cargo to target cells. Drug Deliv 2023;30:2162161. [Crossref] [PubMed]
- Liu C, Xia C, Xia C. Biology and function of exosomes in tumor immunotherapy. Biomed Pharmacother 2023;169:115853. [Crossref] [PubMed]
- Yue M, Hu S, Sun H, et al. Extracellular vesicles remodel tumor environment for cancer immunotherapy. Mol Cancer 2023;22:203. [Crossref] [PubMed]
- Li W, Cai H, Ren L, et al. Sphingosine kinase 1 promotes growth of glioblastoma by increasing inflammation mediated by the NF-κB /IL-6/STAT3 and JNK/PTX3 pathways. Acta Pharm Sin B 2022;12:4390-406. [Crossref] [PubMed]
- Han H, Lee SO, Xu Y, et al. SPHK/HIF-1α Signaling Pathway Has a Critical Role in Chrysin-Induced Anticancer Activity in Hypoxia-Induced PC-3 Cells. Cells 2022;11:2787. [Crossref] [PubMed]
- Chen Z, Liu B. Sphk1 participates in malignant progression of breast cancer by regulating epithelial-mesenchymal transition and stem cell characteristics. Tissue Cell 2020;65:101380. [Crossref] [PubMed]
- Lau P, Zhang G, Zhao S, et al. Sphingosine kinase 1 promotes tumor immune evasion by regulating the MTA3-PD-L1 axis. Cell Mol Immunol 2022;19:1153-67. [Crossref] [PubMed]
- Acharya S, Yao J, Li P, et al. Sphingosine Kinase 1 Signaling Promotes Metastasis of Triple-Negative Breast Cancer. Cancer Res 2019;79:4211-26. [Crossref] [PubMed]
- Hass R, von der Ohe J, Luo T. Human mesenchymal stroma/stem-like cell-derived taxol-loaded EVs/exosomes transfer anti-tumor microRNA signatures and express enhanced SDF-1-mediated tumor tropism. Cell Commun Signal 2024;22:506. [Crossref] [PubMed]


