
Construction and validation of a prognostic model for glioma: an analysis based on mismatch repair-related genes and their correlation with clinicopathological features
Highlight box
Key findings
• EXO1 has been established as an effective tool for the screening, diagnosis, and prognostic assessment of gliomas. Additionally, a nomogram based on the EXO1 risk score was developed and validated to predict the overall survival of glioma patients.
What is known and what is new?
• High EXO1 expression is associated with poor prognosis in various cancers, including non-small cell lung carcinoma, prostate cancer, and hepatocellular carcinoma.
• This study demonstrated that EXO1 is significantly upregulated in glioma and is closely associated with advanced pathological stages, immune cell infiltration, and patient prognosis. These findings suggest its potential as an effective diagnostic marker and therapeutic target for glioma. Additionally, a nomogram incorporating EXO1 risk scores and clinicopathological factors was constructed to comprehensively examine the role of EXO1 in glioma progression. Furthermore, EXO1 protein levels were found to be significantly elevated in patients with glioma.
What is the implication, and what should change now?
• The potential of EXO1 as a predictive biomarker for glioma has been confirmed.
• The efficacy and safety of this nomogram require validation through additional large-scale clinical trials.
Introduction
Gliomas, particularly glioblastoma multiforme (GBM) and lower-grade gliomas (LGG), present significant challenges in neuro-oncology due to their aggressive nature and poor prognoses (1,2). These malignancies are associated with high mortality rates and limited survival times, imposing considerable psychological and economic burdens on patients and their families. Current therapeutic strategies for gliomas—namely surgical excision, radiotherapy, and chemotherapy—often demonstrate limited efficacy because of the tumors’ aggressive behavior and propensity for recurrence (3,4). This situation highlights the urgent need for innovative biomarkers and targeted therapeutic interventions. GBM is the most common type of malignant brain tumor, with an average survival rate of less than 30% over 2 years, despite advancements in treatment options (5-7). The inherent heterogeneity of gliomas, characterized by distinct genetic alterations and variations in histopathological features, complicates diagnostic and treatment strategies. Recent investigations have indicated that significant deficiencies in mismatch repair (MMR) mechanisms, particularly involving mutations in the MSH2, MSH6, and MLH1 genes, are correlated with an elevated tumor mutational burden (TMB) in glioma patients; this association is notably linked to patient survival outcomes (8). In addition, these investigations have revealed that gliomas characterized by MMR deficiencies often display increased microsatellite instability (MSI), a trait that is also connected to treatment efficacy and survival rates (9,10). For instance, a multi-institutional study demonstrated that primary MMR-deficient IDH-mutant astrocytomas are more frequently diagnosed in pediatric and young adult populations, with a median survival duration of merely 15 months (11). However, the specific roles of mismatch repair-related genes (MRRGs) in glioma prognosis remain insufficiently explored (12). This study seeks to address this critical gap by investigating the expression of MRRGs, particularly the EXO1 gene, and its implications for patient outcomes, thereby underscoring the relevance of this research in improving glioma management and therapeutic strategies.
The influence of genetic factors, especially those associated with the MMR system, has garnered increasing attention in glioma research (13,14). Deficiencies in MMR mechanisms can lead to elevated mutational burdens, contributing to tumor development and influencing treatment responses (15,16). For instance, the findings of Cho et al. indicate that MRRGs, including MLH1, MSH2, MSH6, and PMS2, are present even in primary glioblastoma and are associated with high TMB and MGMT promoter methylation (17). This investigation focuses on the expression of MRRGs, particularly the EXO1 gene, in gliomas and its prognostic implications for patients. Previous studies have established a significant correlation between disruptions in the MMR pathway and the progression of various tumors, including gliomas, suggesting that MRRGs may serve as promising biomarkers for disease outcomes (18,19). Our findings indicate that the upregulation of EXO1 and other MRRGs is linked to poor prognoses among glioma patients, highlighting their crucial roles in tumor biology and progression. This groundbreaking insight emphasizes the potential for targeting MRRGs, especially EXO1, in developing novel therapeutic strategies and prognostic tools for glioma management, thus meeting the urgent demand for more effective biomarkers in this aggressive and recurrent malignancy.
This investigation employs a comprehensive methodology that integrates bioinformatics analysis, survival analysis, least absolute shrinkage and selection operator (LASSO) model construction, and assessment of immune cell infiltration to examine the significance of MRRGs in glioma, with particular emphasis on the expression of the EXO1 gene and its prognostic relevance. Gene set analysis has proven to be an effective tool for elucidating the biological implications of gene expression patterns in gliomas, enabling researchers to synthesize data across various studies and derive insights into the involved molecular pathways. Contemporary research increasingly utilizes bioinformatics to analyze extensive genomic datasets, facilitating the identification of critical prognostic indicators. The prognostic relevance of MMR-related genes, especially EXO1, underscores the necessity of understanding their functional roles within the tumor microenvironment. Investigating the interactions between tumor cells and immune cells, such as T lymphocytes and macrophages, could unveil innovative therapeutic strategies, particularly regarding immunotherapy. Moreover, our study specifically focuses on the expression of the EXO1 gene and its implications for prognosis. The strength of this approach lies in its capacity to systematically analyze extensive genomic and clinical databases, thus enhancing the identification of genes correlated with glioma prognosis. Prior investigations have successfully established prognostic models pertinent to gliomas. For instance, Shi et al. devised a prognostic framework grounded in nine ferroptosis-associated long non-coding RNAs (lncRNAs) through the application of LASSO and Cox analyses (20). Likewise, Qu et al. employed both univariate and multivariate Cox regression analyses to discern nine m6A-related gene signatures aimed at forecasting survival probabilities among glioma patients (21). Additionally, Fan et al. formulated an autophagy-related signature, comprising MUL1, NPC1, and TRIM13, utilizing Weighted Gene Co-expression Network Analysis (WGCNA) and Cox regression analyses (22). These prognostic models facilitate the identification of molecular subtypes of gliomas. Nonetheless, our analysis reveals that the applicability of these models is somewhat constrained. Consequently, our research integrates supplementary machine learning algorithms for model development, with a particular focus on the nomogram, which serves to corroborate the efficacy of the EXO1 protein in predicting overall survival (OS) rates at 1 year.
The principal aim of this research is to clarify the prognostic importance of MRRGs in patients with glioma and to establish a predictive model that improves the accuracy of survival forecasts. By employing sophisticated statistical methodologies and thorough data integration, this study aims to provide significant insights into the molecular mechanisms driving glioma progression while identifying potential biomarkers that can inform targeted therapeutic interventions, ultimately striving to enhance clinical outcomes for patients affected by this condition. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2045/rc).
Methods
Data acquisition and processing
A thorough dataset was obtained from The Cancer Genome Atlas (TCGA) database through University of California, Santa Cruz (UCSC) XENA (https://xenabrowser.net/), which encompasses gene sequence data from a total of 706 glioma cases. This collection includes 532 instances of LGG and 174 instances of GBM. This dataset is supplemented by extensive clinical information about the patients. Additionally, gene expression data from 1,140 samples of normal human brain tissue were obtained from the Genotype-Tissue Expression Project (GTEx) database, which can be accessed through UCSC XENA (https://xenabrowser.net/), thereby establishing a strong foundation for comparative analysis.. In addition, a total of twenty-three genes linked to the MMR pathway, collectively designated as MRRGs, were carefully extracted from the KEGG_MISMATCH_REPAIR dataset available in the msigdb database (https://www.gsea-msigdb.org), as specified in Table S1.
Survival analysis
A thorough analysis utilizing the Kaplan-Meier method alongside univariate Cox regression was conducted using the comprehensive “survival” R package (version 3.3.1) on the training cohort (23). The primary objective of these analyses was to systematically identify potential prognostic genes that could provide critical insights into patient survival outcomes and probabilities, thereby enhancing our understanding of survival determinants and informing future therapeutic approaches.
Construction and validation of an MRRGs model
To develop our prognostic model, we employed the LASSO methodology. The penalty parameter was determined with meticulous attention through a rigorous 10-fold cross-validation technique. During this process, we identified 23 candidate MRRGs via LASSO Cox regression analyses, ensuring the robustness and reliability of our findings. Subsequently, a selection of 7 hub genes was made to establish a prognostic risk score model aimed at predicting OS in glioma patients, thereby providing essential insights into patient outcomes and potential therapeutic avenues.
Identification of differentially expressed genes (DEGs)
The gene expression profiles underwent careful normalization and DEGs were identified using the R “DESeq2” package, specifically version 1.36 (24). In this detailed analysis, the criteria for identifying DEGs were stringently set to an absolute log fold change (|logFC|) greater than 2, along with a P value threshold of less than 0.05, ensuring that only the most statistically significant genes were selected for subsequent examination.
Enrichment analyses of Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Set Enrichment Analysis (GSEA)
Using the “clusterProfiler” package (version 4.4.4), comprehensive GO and KEGG pathway enrichment analyses were systematically conducted to elucidate the functional implications of the dataset (25). Additionally, the GSEA software (version 4.1.0) was employed to further explore gene functions related to varying levels of EXO1 expression, particularly focusing on the contrasting profiles of high versus low expression. The analyses adhered to stringent criteria, ensuring that only findings with an adjusted P value below 0.05 and a false discovery rate (FDR) under 0.25 were considered statistically significant, thereby enhancing the credibility and robustness of the results.
Analysis of immune infiltration
To thoroughly assess immune cell infiltration within glioma, a detailed series of evaluations concentrating on this aspect was rigorously conducted. The single-sample GSEA (ssGSEA) method, accessible via the “GSVA” R package (version 1.46), was strategically utilized to evaluate and compare the infiltration levels of various immune cell types between the high-EXO1 and low-EXO1 cohorts, providing valuable insights into the differing immune microenvironments associated with these classifications.
Development of nomograms
Nomograms incorporating clinical characteristics and EXO1 models were developed using the “rms” R package (version 6.3) to predict the OS of glioma specimens based on data from the TCGA cohort. To assess the predictive accuracy of these nomograms, a comprehensive evaluation was conducted utilizing time-dependent calibration curves, facilitating the comparison of predicted outcomes with actual survival data over time. Additionally, univariate Cox regression analysis was performed to determine whether the EXO1 model could serve as an independent prognostic marker for OS in glioma patients. Furthermore, receiver operating characteristic (ROC) curves were utilized to calculate the area under the curve (AUC) value, providing a quantitative assessment of the diagnostic efficacy and utility of the nomogram in predicting patient outcomes.
Patient and tissue specimens
A cohort of 60 patients diagnosed with localized glioma was meticulously recruited from the Department of Pathology at the Second Affiliated Hospital of Zhejiang University School of Medicine. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (No. 2024-0649) and individual consent for this retrospective analysis was waived.
Immunohistochemistry
Paraffin-embedded tissue sections, each 3 µm thick, underwent standard dewaxing and hydration procedures. Antigen retrieval was performed on the specimens, followed by blocking with a 10% goat serum solution for 45 minutes. The samples were subsequently incubated overnight at 4 ℃ with primary antibodies, specifically EXO1 (1:500; HuaBio, Hangzhou, China), MLH1 (1:500; Zhongshan Golden Bridge Biotechnology Co., Beijing, China), MSH2 (1:500; Zhongshan Golden Bridge Biotechnology Co.), MSH6 (1:500; Zhongshan Golden Bridge Biotechnology Co.), and PMS2 (1:500; Zhongshan Golden Bridge Biotechnology Co.). The sections were then exposed to secondary antibodies, namely goat anti-rabbit IgG (1:1,000 dilution; Abcam, Cambridge, UK), for 1 hour. Results were visualized using a diaminobenzidine substrate kit (Abcam), and the sections were counterstained with hematoxylin for 30 seconds. Imaging was conducted using a Nikon light microscope (Nikon, Tokyo, Japan), and the immunohistochemistry results were analyzed with ImageJ software (version 1.8.0).
Statistical analysis
Bioinformatics analyses and R package implementations were performed using R software (version 4.2.0). One-way analysis of variance (ANOVA) was applied to assess differences among multiple groups, followed by Tukey’s post hoc test for pairwise comparisons. For comparisons between the two groups, the Wilcoxon rank-sum test was utilized.
Results
Prognostic model for glioma based on MRRGs
A comparative analysis was conducted to evaluate the expression levels of 23 DNA MRRGs in glioma tissues compared to normal samples. This analysis identified 22 MRRGs that were significantly upregulated in glioma tissue samples (Figure 1A,1B). Furthermore, we investigated the impact of MRRGs expression on glioma patient prognosis. Univariate Cox regression analysis was used to examine the relationship between MRRGs expression and OS in glioma, as depicted in Figure 1C. Analysis of differentially expressed MRRGs demonstrated that 20 of these genes were significantly associated with poorer prognosis in glioma patients.
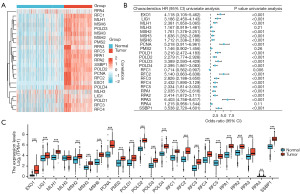
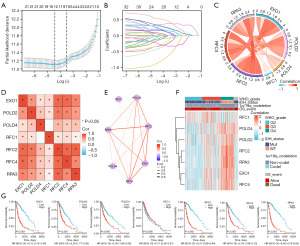
Construction and validation of the prognostic model based on MRRGs
The LASSO algorithm was employed to refine the selection of the 23 candidate genes for constructing the prognostic model (Figure 2A,2B). This process successfully identified seven key genes characterized by the lowest cross-validation error: EXO1, POLD2, POLD4, RFC1, RFC2, RFC4, and RPA3. Subsequent analyses explored the interrelations among these seven pivotal genes in glioma. Utilizing the Spearman correlation method, we observed significant positive correlations among these genes (Figure 2C-2E). The gene expression heatmap, which incorporates clinical parameters, illustrates that elevated expression levels of EXO1, POLD2, POLD4, RFC2, RFC4, and RPA3 are significantly associated with advanced WHO grades, isocitrate dehydrogenase (IDH) wild type, 1p/19q non-codel, and unfavorable prognosis (Figure 2F). The expression levels of these genes not only reflect the biological characteristics of the tumors but also potentially play a crucial role in assessing clinical outcomes. This suggests that future research should prioritize the functional roles of these genes and their mechanisms in tumor progression. Kaplan-Meier survival curves further corroborate the strong association between elevated expression levels of EXO1 (P<0.001), POLD2 (P<0.001), POLD4 (P<0.001), RFC2 (P<0.001), RFC4 (P<0.001), and RPA3 (P<0.001) and poor prognostic outcomes in gliomas. Notably, increased expression of these genes appears to correlate with reduced survival time and disease exacerbation; however, it is intriguing that high expression levels of RFC1 (P=0.006) exhibited an inverse effect (Figure 2G).
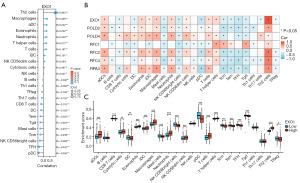
Immune microenvironment landscape of glioma
To enhance our understanding of the immune microenvironment in glioma, we performed an analysis of immune infiltration within the tumor microenvironment. We evaluated immune-related infiltration to assess the Pearson correlation between the immune microenvironment and EXO1 expression using the ssGSEA algorithm. Our findings revealed a positive correlation between EXO1 expression and levels of immune infiltration from Th2 cells (R=0.903), macrophages (R=0.266), activated dendritic cells (aDCs) (R=0.242), eosinophils (R=0.232), neutrophils (R=0.228), T helper cells (R=0.217), T cells (R=0.197), immature dendritic cells (iDCs) (R=0.101), and natural killer (NK) CD56 dim cells (R=0.094). Conversely, we observed a negative correlation between EXO1 expression and regulatory T cells (Tregs) (R=−0.106), Th17 cells (R=−0.120), CD8 T cells (R=−0.176), dendritic cells (DCs) (R=−0.182), effector memory T cells (Tem) (R=−0.220), T gamma delta cells (Tgd) (R=−0.225), mast cells (R=−0.231), central memory T cells (TCM) (R=−0.239), NK CD56bright cells (R=−0.268), T follicular helper (TFH) cells (R=−0.335), and plasmacytoid dendritic cells (pDCs) (R=−0.356) (Figure 3A,3B). Further analysis unveiled significant variations in EXO1 expression levels across various immune cell types (Figure 3C). This investigation also explored different functional subsets of T cells, indicating that EXO1 may play a pivotal role in the immune-inflamed microenvironment of glioma.
GO and KEGG enrichment analysis
We conducted GO and KEGG pathway enrichment analyses to elucidate the biological roles of EXO1. The GO analysis revealed significant enrichment in various biological processes (BP), including pattern specification, anterior/posterior pattern specification, regionalization, chromosome segregation, and nuclear division. Notable cellular components (CC) identified included the kinetochore, chromosomal region, and DNA packaging complex. The molecular functions (MF) exhibited significant enrichment for extracellular matrix structural constituents, DNA-binding transcription activator activity, and glycosaminoglycan binding. The findings from the KEGG pathway enrichment analysis highlighted significant enrichment in pathways related to the cell cycle, transcriptional misregulation in cancer, systemic lupus erythematosus, alcoholism, and the formation of neutrophil extracellular traps (Figure 4, Table 1). Together, these results underscore the important role of EXO1 in glioma progression.
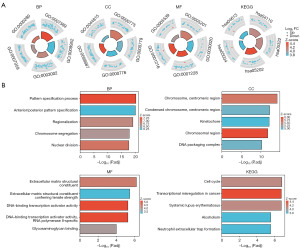
Table 1
| Ontology | ID | Description | P value |
|---|---|---|---|
| BP | GO:0007389 | Pattern specification process | 2.14e−24 |
| GO:0009952 | Anterior/posterior pattern specification | 6.1e−24 | |
| GO:0003002 | Regionalization | 7.68e−23 | |
| GO:0007059 | Chromosome segregation | 2.11e−21 | |
| GO:0000280 | Nuclear division | 4.66e−21 | |
| CC | GO:0000775 | Chromosome, centromeric region | 1.94e−17 |
| GO:0000779 | Condensed chromosome, centromeric region | 3.24e−15 | |
| GO:0000776 | Kinetochore | 6.5e−15 | |
| GO:0098687 | Chromosomal region | 1.99e−14 | |
| GO:0044815 | DNA packaging complex | 8.72e−13 | |
| MF | GO:0005201 | Extracellular matrix structural constituent | 1.27e−09 |
| GO:0030020 | Extracellular matrix structural constituent conferring tensile strength | 1.38e−08 | |
| GO:0001228 | DNA-binding transcription activator activity, RNA polymerase II-specific | 3.6e−08 | |
| GO:0001216 | DNA-binding transcription activator activity | 4.38e−08 | |
| GO:0005539 | Glycosaminoglycan binding | 1.04e−06 | |
| KEGG | hsa04110 | Cell cycle | 6.27e−10 |
| hsa05322 | Systemic lupus erythematosus | 2.1e−09 | |
| hsa05202 | Transcriptional misregulation in cancer | 2.13e−09 | |
| hsa05034 | Alcoholism | 4.58e−08 | |
| hsa04613 | Neutrophil extracellular trap formation | 5.86e−08 |
BP, biological processes; CC, cellular components; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; MF, molecular functions.
GSEA enrichment analysis
To assess the impact of high and low EXO1 expression on glioma progression, we conducted a GSEA to identify significantly enriched pathways. The analysis revealed that the high-EXO1 group was notably enriched in processes including interleukin (IL) signaling, transcriptional regulation by TP53, the PI3K-Akt signaling pathway, IL-18 signaling pathway, mitotic G2/M phases, DNA repair, M phase, and cell cycle checkpoints (Figure 5).

Association of EXO1 expression with clinical parameters in glioma
We observed that EXO1 expression increased with advancing tumor stage. Elevated EXO1 levels were positively correlated with high WHO grading (P<0.001), IDH wild-type status (P<0.001), 1p/19q non-codeletion status (P<0.001), and patient age (P<0.001) (Figure 6A-6D). Conversely, increased EXO1 expression was associated with poorer prognostic outcomes (P<0.001) (Figure 6E). ROC curve analysis further confirmed the robust performance of our risk model, with AUC values of 0.859, 0.804, 0.654, and 0.681 (Figure 6F-6I). Additionally, among patients with IDH mutations (P=0.02) and 1p/19q non-co-deletion (P<0.001), those with lower EXO1 expression exhibited a significant survival advantage (Figure 6J-6M). Time-dependent ROC curves demonstrated AUC values for 1, 3, and 5 years exceeding 0.75, indicating strong model performance (Figure 6N). We subsequently integrated WHO grade, IDH status, 1p/19q co-deletion, gender, age, and EXO1 expression to construct a nomogram for survival prediction (Figure 6O). The calibration chart revealed that effectiveness of the nomogram was accurate (Figure 6P) (c-index =0.850). Notably, EXO1 expression has the potential to enhance the accuracy of survival probability predictions at the 1-year intervals. Overall, a significant correlation was confirmed between EXO1 expression levels and prognostic outcomes in glioma, with consistent results from the Chi-squared test (Table 2).
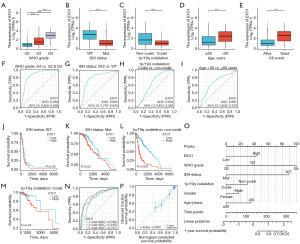
Table 2
| Variables | Low expression of EXO1 (n=348) | High expression of EXO1 (n=351) | P value |
|---|---|---|---|
| WHO grade (n=637) | <0.001 | ||
| G2 | 183 (28.7) | 41 (6.4) | |
| G3 | 112 (17.6) | 133 (20.9) | |
| G4 | 12 (1.9) | 156 (24.5) | |
| IDH status (n=689) | <0.001 | ||
| WT | 43 (6.2) | 203 (29.5) | |
| Mut | 302 (43.8) | 141 (20.5) | |
| 1p/19q codeletion (n=692) | <0.001 | ||
| Non-codel | 225 (32.5) | 295 (42.6) | |
| Codel | 122 (17.6) | 50 (7.2) | |
| OS event (n=699) | <0.001 | ||
| Alive | 285 (40.8) | 142 (20.3) | |
| Dead | 63 (9) | 209 (29.9) | |
| DSS event (n=678) | <0.001 | ||
| No | 286 (42.2) | 148 (21.8) | |
| Yes | 56 (8.3) | 188 (27.7) | |
| PFI event (n=699) | <0.001 | ||
| No | 235 (33.6) | 118 (16.9) | |
| Yes | 113 (16.2) | 233 (33.3) |
Data are presented as n (%). DSS, disease-specific survival; IDH, isocitrate dehydrogenase; Mut, mutant; OS, overall survival; PFI, progression-free interval; WT, wild type; WHO, World Health Organization.
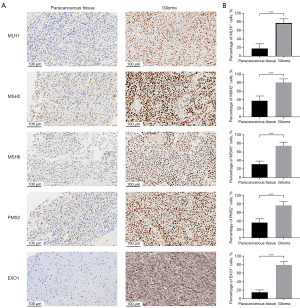
Validation of MMR protein expression
In this comprehensive study, we meticulously investigated the expression differences of the EXO1 protein between glioma tissues and the adjacent non-cancerous tissues, aiming to uncover potential biomarkers for this aggressive form of brain cancer. Our assessment extended beyond EXO1 to include a thorough examination of other crucial MMR proteins, specifically MLH1, MSH2, MSH6, and PMS2, employing immunohistochemical techniques to ensure precise and reliable results. The findings from our analysis revealed that the expression levels of EXO1 (P<0.001), along with MLH1 (P<0.001), MSH2 (P<0.001), MSH6 (P<0.001), and PMS2 (P<0.001), were significantly elevated in glioma tissues when compared to the surrounding paracancerous tissues, as illustrated in Figure 7, highlighting the potential role of these proteins in the pathology of gliomas.
Discussion
Gliomas present a significant challenge in neuro-oncology due to their aggressive nature and unfavorable prognostic outcomes (26,27). This type of malignancy is associated with serious health concerns, as reflected in high mortality rates and limited survival periods, which impose substantial psychological and financial burdens on patients and their families (28,29). Among gliomas, GBM is the most common and aggressive form of primary brain tumor, accounting for nearly 50% of all cases and exhibiting a stark 5-year survival rate of less than 10% (30-33). Current therapeutic strategies, including surgical interventions, radiotherapy, and chemotherapy, often show limited effectiveness because of the tumor’s aggressive behavior and tendency to recur. This situation underscores the urgent need for innovative biomarkers and targeted therapeutic approaches. The complex nature of glioma pathology, characterized by various tumor types and distinct molecular profiles, complicates diagnostic and therapeutic strategies, further emphasizing the necessity for viable biomarkers and targeted treatments (34-36).
The research methodologies employed in this investigation demonstrate notable strengths through comprehensive integration of bioinformatics analyses, survival evaluations, and assessments of immune infiltration, collectively enhancing the robustness of our findings. By leveraging extensive genomic datasets alongside clinical data, we successfully identified and validated the prognostic relevance of MRRGs in glioma. This multifaceted approach highlights the potential of MRRGs, particularly EXO1, as promising biomarkers and therapeutic targets, thereby paving the way for future research initiatives aimed at improving clinical outcomes for glioma patients.
The significant positive correlations observed among these genes suggest a coordinated regulatory network that may influence tumor progression and patient prognosis. Such interactions are crucial, as they could inform the development of therapeutic strategies targeting these pathways. For instance, the elevated expression of these genes in glioma tissues may indicate an enhanced capacity for DNA repair, which is often associated with the tumor’s resilience to standard treatments. This finding aligns with previous research highlighting the role of MRRGs in maintaining genomic stability and their potential implications for tumor aggressiveness (37,38).
Furthermore, our analysis of immune infiltration revealed a complex interplay between EXO1 expression and various immune cell populations within the glioma microenvironment. Notably, increased levels of EXO1 were associated with a higher presence of Th2 cells and macrophages, while showing an inverse correlation with the infiltration of CD8+ T cells. This suggests a possible immune evasion strategy mediated by EXO1, which may contribute to the poor prognosis observed in glioma patients. Recent studies have emphasized the importance of the interaction between tumor cells and the immune microenvironment in understanding tumor progression and treatment resistance (39-41). Thus, there is a pressing need to further investigate how MRRGs, such as EXO1, can be strategically targeted to enhance immune responses in glioma therapy.
The findings from our enrichment analysis revealed significant correlations between EXO1 expression and several key biological pathways, particularly those regulating the cell cycle, transcriptional dysregulation in cancer, and the PI3K-Akt signaling pathway. The cell cycle pathway is essential for regulating cellular proliferation and maintaining genomic stability; its disruption is a hallmark of cancer, leading to uncontrolled cell division and tumor progression (42,43). Additionally, the pathway associated with transcriptional misregulation in cancer highlights the importance of gene expression regulation in tumorigenesis. Abnormal transcriptional control can activate oncogenes and repress tumor suppressor genes, thereby promoting cancer progression (44). The notable enrichment of this pathway in our study underscores the potential of EXO1 as a modulator of transcriptional networks in glioma, warranting further investigation into its mechanistic roles in gene expression alterations. Finally, the PI3K-Akt signaling pathway is a critical regulator of various cellular functions, including metabolism, growth, and survival. Its activation is commonly observed in multiple cancer types, including gliomas, and is associated with poor clinical outcomes (45). Collectively, these pathways elucidate the multifaceted role of EXO1 in glioma progression and highlight its potential as both a biomarker and a therapeutic target in clinical settings.
The validation of EXO1 expression through immunohistochemical analysis provides substantial evidence for its role in glioma pathology. Our investigation demonstrates that EXO1 levels are significantly elevated in glioma tissues compared to adjacent normal tissues, reinforcing bioinformatics data that indicate a strong correlation between EXO1 and various clinical parameters. Moreover, EXO1 may influence glioma progression through its involvement in DNA repair processes. The observed association between EXO1 and several MMR genes—including MLH1, MSH2, MSH6, and PMS2—underscores the potential significance of EXO1 as a critical component of the MMR pathway, which is essential for maintaining genomic integrity and influencing tumor development. The notable upregulation of EXO1 in glioma specimens aligns with previous research highlighting the critical role of MMR genes in cancer progression and patient prognosis (46,47). Furthermore, integrating immunohistochemical results with bioinformatics assessments enhances the credibility of our predictive model, which demonstrates strong efficacy in survival forecasting. This model, which combines EXO1 expression with other clinical variables, has the potential to inform clinical decision-making and personalize treatment approaches for glioma patients. The implications of these findings are significant, as they not only deepen our understanding of the molecular mechanisms driving glioma but also suggest that EXO1 could serve as a valuable biomarker for patient stratification and prognostic evaluation.
The limitations of this study primarily relate to the lack of comprehensive validation through wet laboratory experiments, which may undermine the robustness of our results. Additionally, the relatively small sample size could affect the generalizability of the findings, as a larger cohort might provide more conclusive insights into the prognostic relevance of the identified MRRGs. These considerations underscore the necessity for further validation and investigation in larger, more heterogeneous populations to confirm the clinical relevance of our prognostic model. Subsequent investigations ought to integrate experimental laboratory work to confirm the functional roles of these genes, thereby offering a clearer comprehension of the fundamental BP involved. Furthermore, future research should aim to include larger and more varied sample cohorts to improve the reliability and applicability of the model.
In summary, this study highlights the significant contributions of EXO1 and other MRRGs to glioma prognosis, establishing a foundation for future research and clinical applications. Moreover, the developed prognostic model exhibits strong predictive capabilities, which could enhance clinical decision-making and improve patient stratification. Future investigations should focus on validating these findings in larger cohorts and exploring the therapeutic implications of targeting EXO1 and associated pathways, ultimately aiming to improve outcomes for patients with glioma.
Conclusions
This investigation effectively integrates macroscopic and microscopic methodologies to develop a comprehensive screening approach for MRRGs. Utilizing big data analytics, bioinformatics, and histopathological examination, the study ensures a detailed and efficient analysis of MRRGs. The findings indicate that increased expression of EXO1 in glioma is associated with disease progression and poor prognosis. Moreover, EXO1 protein levels were significantly higher in individuals diagnosed with glioma. Identifying EXO1 may enhance personalized treatment strategies for glioma patients and aid in optimizing therapeutic decisions.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2045/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2045/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2045/prf
Funding: This work was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2045/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the institutional ethics committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (No. 2024-0649) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benmelouka AY, Munir M, Sayed A, et al. Neural Stem Cell-Based Therapies and Glioblastoma Management: Current Evidence and Clinical Challenges. Int J Mol Sci 2021;22:2258. [Crossref] [PubMed]
- Seyhan AA. Circulating Liquid Biopsy Biomarkers in Glioblastoma: Advances and Challenges. Int J Mol Sci 2024;25:7974. [Crossref] [PubMed]
- Zhuang D, Zhang H, Hu G, et al. Recent development of contrast agents for magnetic resonance and multimodal imaging of glioblastoma. J Nanobiotechnology 2022;20:284. [Crossref] [PubMed]
- Nyati KK, Kishimoto T. The emerging role of Arid5a in cancer: A new target for tumors. Genes Dis 2023;10:813-24. [Crossref] [PubMed]
- Cetin MH, Rieckmann T, Hoffer K, et al. G2 checkpoint targeting via Wee1 inhibition radiosensitizes EGFRvIII-positive glioblastoma cells. Radiat Oncol 2023;18:19. [Crossref] [PubMed]
- Vézina A, Manglani M, Morris D, et al. Adenosine A2A Receptor Activation Enhances Blood-Tumor Barrier Permeability in a Rodent Glioma Model. Mol Cancer Res 2021;19:2081-95. [Crossref] [PubMed]
- Chalise L, Kato A, Ohno M, et al. Efficacy of cancer-specific anti-podoplanin CAR-T cells and oncolytic herpes virus G47Δ combination therapy against glioblastoma. Mol Ther Oncolytics 2022;26:265-74. [Crossref] [PubMed]
- Suwala AK, Stichel D, Schrimpf D, et al. Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol 2021;141:85-100. [Crossref] [PubMed]
- Chen L, Zhao X, Liu Y, et al. Comprehensive analysis of HHV-6 and HHV-7-related gene signature in prognosis and response to temozolomide of glioma. J Med Virol 2023;95:e28285. [Crossref] [PubMed]
- Hunter C, Smith R, Cahill DP, et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res 2006;66:3987-91. [Crossref] [PubMed]
- Noll A, Myers C, Biery MC, et al. Therapeutic HDAC inhibition in hypermutant diffuse intrinsic pontine glioma. Neoplasia 2023;43:100921. [Crossref] [PubMed]
- Cheng X, An J, Lou J, et al. Trans-lesion synthesis and mismatch repair pathway crosstalk defines chemoresistance and hypermutation mechanisms in glioblastoma. Nat Commun 2024;15:1957. [Crossref] [PubMed]
- Guerrini-Rousseau L, Merlevede J, Denizeau P, et al. Glioma oncogenesis in the Constitutional mismatch repair deficiency (CMMRD) syndrome. Neurooncol Adv 2024;6:vdae120. [Crossref] [PubMed]
- Palova H, Das A, Pokorna P, et al. Precision immuno-oncology approach for four malignant tumors in siblings with constitutional mismatch repair deficiency syndrome. NPJ Precis Oncol 2024;8:110. [Crossref] [PubMed]
- Marin JJG, Sanchon-Sanchez P, Cives-Losada C, et al. Novel Pharmacological Options in the Treatment of Cholangiocarcinoma: Mechanisms of Resistance. Cancers (Basel) 2021;13:2358. [Crossref] [PubMed]
- Song Y, Kerr TD, Sanders C, et al. Organoids and metastatic orthotopic mouse model for mismatch repair-deficient colorectal cancer. Front Oncol 2023;13:1223915. [Crossref] [PubMed]
- Cho YA, Kim D, Lee B, et al. Incidence, clinicopathologic, and genetic characteristics of mismatch repair gene-mutated glioblastomas. J Neurooncol 2021;153:43-53. [Crossref] [PubMed]
- Wang W, Li T, Xie Z, et al. Integrating single-cell and bulk RNA sequencing data unveils antigen presentation and process-related CAFS and establishes a predictive signature in prostate cancer. J Transl Med 2024;22:57. [Crossref] [PubMed]
- Jung KW, Jung JH, Park HY. Functional Roles of Homologous Recombination and Non-Homologous End Joining in DNA Damage Response and Microevolution in Cryptococcus neoformans. J Fungi (Basel) 2021;7:566. [Crossref] [PubMed]
- Shi J, Lai D, Zuo X, et al. Identification of Ferroptosis-Related Biomarkers for Prognosis and Immunotherapy in Patients With Glioma. Front Cell Dev Biol 2022;10:817643. [Crossref] [PubMed]
- Qu S, Chen Z, Liu B, et al. N6-methyladenine-related genes affect biological behavior and the prognosis of glioma. Cancer Med 2021;10:98-108. [Crossref] [PubMed]
- Fan Y, Peng X, Li B, et al. Development of Autophagy Signature-Based Prognostic Nomogram for Refined Glioma Survival Prognostication. Biomed Res Int 2020;2020:1872962. [Crossref] [PubMed]
- Liu J, Lichtenberg T, Hoadley KA, et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018;173:400-416.e11. [Crossref] [PubMed]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [Crossref] [PubMed]
- Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284-7. [Crossref] [PubMed]
- Chen S, Jiang Y, Wang C, et al. Epigenetic clocks and gliomas: unveiling the molecular interactions between aging and tumor development. Front Mol Biosci 2024;11:1446428. [Crossref] [PubMed]
- Wang W, Ou Z, Huang X, et al. Microbiota and glioma: a new perspective from association to clinical translation. Gut Microbes 2024;16:2394166. [Crossref] [PubMed]
- Ross JL, Puigdelloses-Vallcorba M, Piñero G, et al. Microglia and monocyte-derived macrophages drive progression of pediatric high-grade gliomas and are transcriptionally shaped by histone mutations. Immunity 2024;57:2669-2687.e6. [Crossref] [PubMed]
- Gallitto M, Zhang X, De Los Santos G, et al. Targeted delivery of napabucasin with radiotherapy improves outcomes in diffuse midline glioma. Neuro Oncol 2025;27:795-810. [Crossref] [PubMed]
- Liu HJ, Xu P. Strategies to overcome/penetrate the BBB for systemic nanoparticle delivery to the brain/brain tumor. Adv Drug Deliv Rev 2022;191:114619. [Crossref] [PubMed]
- Davy M, Genest L, Legrand C, et al. Evaluation of Temozolomide and Fingolimod Treatments in Glioblastoma Preclinical Models. Cancers (Basel) 2023;15:4478. [Crossref] [PubMed]
- García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, et al. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials (Basel) 2019;9:638. [Crossref] [PubMed]
- Magalhaes YT, Boell VK, Cardella GD, et al. Downregulation of the Rho GTPase pathway abrogates resistance to ionizing radiation in wild-type p53 glioblastoma by suppressing DNA repair mechanisms. Cell Death Dis 2023;14:283. [Crossref] [PubMed]
- Andrew Awuah W, Shah MH, Tan JK, et al. Immunotherapeutic advances in glioma management: The rise of vaccine-based approaches. CNS Neurosci Ther 2024;30:e70013. [Crossref] [PubMed]
- Yang XL, Shi Y, Zhang DD, et al. Quantitative proteomics characterization of cancer biomarkers and treatment. Mol Ther Oncolytics 2021;21:255-63. [Crossref] [PubMed]
- Lin Y, Li H, Ge Q, et al. Establishment and validation of a prognostic prediction model for glioma based on key genes and clinical factors. Transl Cancer Res 2025;14:240-53. [Crossref] [PubMed]
- Xie T, Feng Q, Li Z, et al. Heterogeneous constitutional mismatch repair deficiency with MSH6 missense mutation clinically benefits from pembrolizumab and regorafenib combination therapy: a case report and literature review. Hered Cancer Clin Pract 2021;19:7. [Crossref] [PubMed]
- Zhang Y, Wang S, Han S, et al. Pan-Cancer Analysis Based on EPOR Expression With Potential Value in Prognosis and Tumor Immunity in 33 Tumors. Front Oncol 2022;12:844794. [Crossref] [PubMed]
- Zhao H, Ming T, Tang S, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer 2022;21:144. [Crossref] [PubMed]
- Gao D, Zhou L, Bao Y, et al. Novel cuprotosis-related gene signature: a prognostic indicator and regulator of the glioma immune microenvironment. Transl Cancer Res 2024;13:6282-97. [Crossref] [PubMed]
- He J, Guo J, Sun P. Prognostic value of CTF1 in glioma and its role in the tumor microenvironment. Transl Cancer Res 2024;13:6862-79. [Crossref] [PubMed]
- Zhang JY, Xu D, Liu ZZ, et al. Human U Three Protein 14a Expression is Increased in Hepatocellular Carcinoma and Associated with Poor Prognosis. Chin Med J (Engl) 2017;130:470-6. [Crossref] [PubMed]
- Baker AM, Cross W, Curtius K, et al. Evolutionary history of human colitis-associated colorectal cancer. Gut 2019;68:985-95. [Crossref] [PubMed]
- Zhong F, Chen T, Li B. Combinatorial transcriptional regulation of HEB/ZEB1/ASCL1 and MYBL2 on Ras/ErbB signaling. Biochem Biophys Res Commun 2022;622:170-6. [Crossref] [PubMed]
- Zheng SX, Chen JP, Liang RS, et al. Schizophyllum commune fruiting body polysaccharides inhibit glioma by mediating ARHI regulation of PI3K/AKT signalling pathway. Int J Biol Macromol 2024;279:135326. [Crossref] [PubMed]
- Xu H, Chai S, Wang Y, et al. Molecular and clinical characterization of PARP9 in gliomas: A potential immunotherapeutic target. CNS Neurosci Ther 2020;26:804-14. [Crossref] [PubMed]
- Jaworski D, Brzoszczyk B, Szylberg Ł. Recent Research Advances in Double-Strand Break and Mismatch Repair Defects in Prostate Cancer and Potential Clinical Applications. Cells 2023;12:1375. [Crossref] [PubMed]


