
CREB3-mediated upregulation of MIR210HG transcription enhances proliferation in colon cancer cells
Highlight box
Key findings
• The study identifies the interaction between long non-coding RNA (lncRNA) MIR210HG and the transcription factor cyclic adenosine monophosphate-responsive element-binding protein 3 (CREB3) in colon cancer (CC), demonstrating their regulatory influence on CC cell proliferation.
What is known and what is new?
• It is known that lncRNAs play critical roles in cancer biology, influencing various cellular processes including proliferation and metastasis.
• This study provides new insights by revealing that the CREB3-MIR210HG axis is a significant regulatory mechanism in CC, suggesting a novel therapeutic target for cc treatment through modulation of this pathway.
What is the implication, and what should change now?
• Targeting the CREB3-MIR210HG axis may offer new strategies for CC therapy. Further investigations are needed to explore the potential of lncRNA-based interventions in clinical settings, aiming to improve treatment outcomes for CC patients.
Introduction
The role of non-coding RNAs in tumor initiation and progression has been increasingly elucidated in recent years. Long non-coding RNAs (lncRNAs) are a class of RNA molecules with a length >200 nucleotides (1,2). Despite not encoding proteins, this class of RNA plays a crucial role in gene expression regulation (3,4), cellular differentiation (5,6), and the development of various diseases, including cancer (7,8). Growing evidence suggests that lncRNAs, through interactions with DNA, RNA or proteins, are involved in key processes of cancer development, such as uncontrolled proliferation, evasion of growth inhibition, promotion of angiogenesis and tissue metastasis (9,10).
Among the extensively studied lncRNAs, MIR210HG has attracted particular attention. Its abnormal expression in various cancer diseases is closely associated with malignant behaviors of tumor cells (11-13). As an important molecule regulating the dynamic tumor microenvironment (14,15) and influencing tumor biology, the aberrant expression of MIR210HG has been confirmed to be closely related to tumor invasiveness, metastatic potential and patient prognosis, implying its potential as a target for future cancer diagnosis and treatment (13,16).
However, the expression of lncRNAs itself is a highly regulated process and upstream regulatory mechanisms play a decisive role in the stability and functional role of lncRNAs (17). Multiple mechanisms, including transcription factors, epigenetic modifications and binding of RNA polymerase, collectively influence the expression patterns of lncRNAs (18-20). Despite the growing understanding of the role of MIR210HG in various cancers, there have been few studies on its upstream regulatory mechanisms. This knowledge gap limits the comprehensive understanding of the functional role of MIR210HG in diseases such as colon cancer (CC) and hinders the investigation of its potential as a clinical therapeutic target. Therefore, unraveling the mystery of MIR210HG expression regulation holds significant importance in improving the diagnosis and treatment of CC and may provide new strategies for cancer therapy. We present this article in accordance with the MDAR reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1525/rc).
Methods
Database utilization
The genomic localization of MIR210HG was determined using the National Center for Biotechnology Information (NCBI) Gene database (https://www.ncbi.nlm.nih.gov/gene). The potential promoter region was characterized based on the established criterion of 2,000 base pairs (bp) upstream and 100 bp downstream of the transcription start site (TSS), as annotated in the human genome reference sequence.
Predictive analysis for transcription factor binding sites within the MIR210HG promoter region was conducted using The University of California Santa Cruz (UCSC) Genome Browser (http://genome.ucsc.edu/) and the JASPAR database (http://jaspar.genereg.net). The sequence encompassing the putative promoter region was input into these databases to identify potential transcription factor cyclic adenosine monophosphate (AMP)-responsive element-binding protein 3 (CREB3) binding motifs.
Expression data for MIR210HG and CREB3 in CC tissues were extracted from The Cancer Genome Atlas (TCGA) database. Samples were categorized into low and high-expression groups (Lower/Higher Group) based on CREB3 levels. Comparative analysis of MIR210HG expression between these groups was performed using appropriate statistical tests. Co-expression of CREB3 and MIR210HG in CC was determined using the starBase v3.0 database (https://rnasysu.com/encori/). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Cell culture and gene manipulation
The human CC cell lines SW480 and SW620 were cultured under standard conditions and were purchased from Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (China). Human Embryonic Kidney 293T Cells (HEK293T) cells were also maintained for promoter activity assays.
CREB3 overexpressing (LV-CREB3) and knockdown (sh-CREB3) lentiviruses and corresponding control viruses (LV-NC) were purchased from Shanghai GeneChem Co., Ltd. (China). MIR210HG knockdown antisense oligonucleotides were purchased from Guangzhou RiboBio Co., Ltd. (China). CREB3 overexpression and knockdown in CC cells were achieved through transfection with specific lentiviruses and short hairpin RNA (shRNA), respectively. The sequences used were as follows: sh-NC: TTCTCCGAACGTGTCACGT; sh-CREB3: UUUCUGAGCAGUGUAUCAUAUUGGG.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using a standardized protocol, followed by reverse transcription to synthesize complementary DNA. Expression levels of MIR210HG and CREB3 were quantified using RT-qPCR with specific primers and probes. Data normalization was conducted using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. MIR210HG primers forward: TGTTCCCTTTGTGTGCTCCAG, reverse: GCCCTAGATCATGGGGTCTT; CREB3 primers forward: CCCACCCTTTCCGTAGTTGT, reverse: CTCGGTACCTCAGAAAGCGG; GAPDH primers forward: GCACCGTCAAGGCTGAGAAC, reverse: TGGTGAAGACGCCAGTGG.
Western blot
To perform a Western blot, prepare a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel based on the target protein’s molecular weight, then assemble and fill the electrophoresis apparatus. Load 5 µL of pre-stained markers and 25 µg of sample into wells, and run electrophoresis at 100 V. Transfer proteins to an NC membrane using wet transfer at 280 mA for 75–120 min. Block the membrane for 10 min, then cut into strips by molecular weight. Incubate strips in primary antibody overnight at 4 ℃, then wash twice with ris-Buffered Saline with Tween 20 (TBST) for 10 min each. Incubate in secondary antibody for 1 hour at room temperature (RT), followed by two more TBST washes. Mix equal parts of solutions A and B to create an enhanced chemiluminescence (ECL) reagent (Millipore, USA) working solution, apply to strips, and visualize using a gel imaging system. CREB3 (ab180119, 1:1,000, Abcam, Cambridge, UK), GAPDH [5174, 1:5,000, Cell Signaling Technology (CST), Danvers, MA, USA] antibodies were used in this study.
Promoter activity assay
Wild-type (WT) MIR210HG promoter variants were cloned into luciferase reporter vectors. HEK293T cells were transfected with the constructed plasmids and promoter activity was assessed by measuring luciferase activity post-transfection.
Cells were seeded into 24-well plates 1 day before transfection to reach a cell density of 70–80% at the time of transfection. The cells were cultured in a Dulbecco’s modified Eagle medium (DMEM; Gibco, Carlsbad, USA) medium. The plasmid was added to 50 µL of serum-free DMEM medium at a ratio of promoter + transcription factor: pGL3-Basic: phRL-TK (Renilla luciferase reporter plasmid) (TK) = 10:1, with a total plasmid amount of 2 µg. The mixture was incubated at RT for 5 min. Subsequently, 2 µL of Lipofectamine 2000™ transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, USA) was added to 50 µL of serum-free DMEM medium and the two solutions were combined and incubated at RT for 15 min. Finally, serum-free DMEM medium was added to bring the total volume to 500 µL. Finally, 500 µL of the pre-prepared transfection complex was incorporated and incubated at 37 ℃ for 4–6 h.
Chromatin immunoprecipitation (ChIP)
The ChIP assay was carried out by cross-linking approximately 1×107 cells in 1% formaldehyde for 10 min at RT to stabilize the interactions between DNA and proteins. The cross-linking reaction was then quenched by the addition of glycine, followed by washing of the cells with ice-cold phosphate-buffered saline. Subsequently, cell lysis was achieved using ChIP Lysis Buffer supplemented with protease inhibitors and the DNA was fragmented to sizes ranging from 100–500 bp through sonication. Finally, debris was removed via centrifugation. Immunoprecipitation (IP) entailed the dilution of sonicated lysates with TBS and subsequent incubation with specific antibodies or control immunoglobulin G (IgG) overnight at 4 ℃. Protein G Agarose beads, pre-blocked with salmon sperm DNA, were introduced to facilitate the binding of the immune complexes. Following incubation, the beads underwent washing with assorted salt buffers and Tris-EDTA (TE) buffer, before being eluted with ChIP Elution Buffer at 65 ℃. The input controls were calibrated to achieve uniform volume and processed in conjunction with the IP samples. After elution, both IP and input samples underwent reverse cross-linking by adding NaCl and overnight incubation at 65 ℃. Digestion of RNA and proteins was carried out using RNase A and proteinase K treatments. Subsequently, DNA was isolated using a conventional purification kit for further analysis.
Cell viability and proliferation
Cell viability and proliferation were assessed using the Cell Counting Kit-8 (CCK-8; Dojindo, Japan) and 5-ethynyl-2’-deoxyuridine (EdU) assay to investigate the impact of manipulating CREB3 and MIR210HG expression.
For the CCK-8 assay, cell seeding in 96-well plates and overnight adhesion, transfections were conducted to modulate the expression levels of the target genes. After incubation for gene expression modulation, CCK-8 reagent was added to the wells and cells were further incubated for 1–4 h to facilitate the conversion of WST-8 to formazan by active dehydrogenases. The absorbance of formazan was quantified at 450 nm, utilizing cell-free wells as controls to account for background noise. Comparative analysis of absorbance values between treated and control cells yielded data on cell proliferation. The experiments were performed in triplicate and repeated to ensure the consistency and reliability of the findings.
To further validate the findings, an EdU incorporation assay was conducted to assess cell proliferation. Cells were seeded into Coverglass Bottom Dish at a density of 1,000 cells per well and cultured for 48 hours. Subsequently, cells were incubated with EdU (10 µM) for 2 hours at 37 ℃. After incubation, cells were fixed with 4% paraformaldehyde at RT for 10 min. Cell nuclei were then stained with 4’,6-diamidino-2-phenylindole (DAPI) (5 mg/mL; Beyotime Institute of Biotechnology, Shanghai, China) for 10 min at RT. EdU-positive cells were visualized and quantified using a laser confocal microscope with a magnification of ×200. The proportion of EdU-positive cells was calculated to provide additional evidence of cell proliferation.
Statistical analysis
All experiments were performed triplicated. Differences between two groups were evaluated using the Student t-test. P<0.05 was considered to indicate a statistically significant difference. Data were presented as mean ± standard deviation.
Results
Identification of the MIR210HG promoter region
The MIR210HG gene is allocated on the antisense strand of chromosome 11, specifically at coordinates 11q13.1 (Chr11: 565,657–568,457), according to the NCBI Gene repository. The present investigation into the regulatory machinations of gene expression in CC identified the critical promoter region of MIR210HG, conventionally encompassing 2,000 bp upstream and 100 bp downstream of the TSS. For MIR210HG, this pertinent region was discerned at Chr11: 568,358–570,457, considering the gene orientation and reverse transcription.
In silico analysis via the UCSC Genome Browser facilitated the recognition of transcription factor binding sites within this promoter region. Notably, utilizing the JASPAR database to examine the sequence led to the identification of putative binding sites for transcription factor CREB3 (Table 1), offering insights into the regulatory dynamics of MIR210HG expression in CC.
Table 1
| ID | Name | Score | Relative score | Sequence ID | Start | End | Strand | Predicted sequence |
|---|---|---|---|---|---|---|---|---|
| MA0638.1 | CREB3 | 11.52 | 0.86 | NC_000011.10:c570457-568358 | 439 | 452 | + | GGTTCACGTCAGCA |
| MA0638.1 | CREB3 | 9.58 | 0.83 | NC_000011.10:c570457-568358 | 1,094 | 1,107 | + | CTTCCACGTCTGAA |
CREB3, cyclic adenosine monophosphate-responsive element-binding protein 3.
Validation of CREB3 regulatory influence on MIR210HG
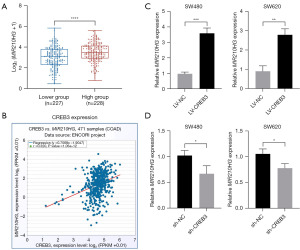
This study utilized TCGA to analyze the gene expression in 455 CC samples. Stratification by CREB3 expression yielded two cohorts: One exhibiting low expression (N=227); and another with high expression (N=228). Statistical evaluation showed a significant upregulation of MIR210HG in the high CREB3 cohort (Figure 1A). Moreover, the starBase v3.0 platform confirmed a significant co-expression correlation between CREB3 and MIR210HG in CC, suggesting a potential interactive and regulatory association pertinent to the disease molecular pathology (Figure 1B). Experimental manipulations of CREB3 expression in SW480 and SW620 cells, including overexpression and knockdown, were quantified via RT-qPCR. Data demonstrated that MIR210HG levels positively correlated with CREB3 expression (Figure 1C), while CREB3 suppression led to decreased MIR210HG mRNA (Figure 1D). These observations imply a putative activating regulatory role for CREB3 on MIR210HG expression, with further empirical studies required for conclusive mechanistic elucidation.
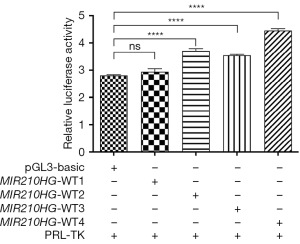
Promoter activity assay
Following transfection with MIR210HG promoter constructs WT1, WT2, WT3 and WT4, only WT2, WT3 and WT4 demonstrated a marked increase in reporter activity in HEK293T cells compared with the control vector, pGL3-basic (Figure 2). This indicates that specific variants of the MIR210HG promoter can significantly activate transcription.
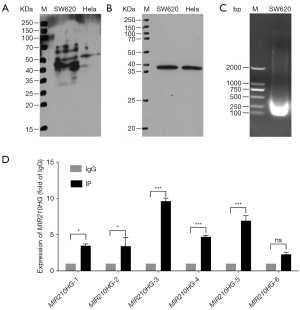
Validation of CREB3 and MIR210HG promoter interaction
ChIP assays substantiated the presence of CREB3 binding sites within the MIR210HG promoter. Western blot analysis confirmed the specificity of the CREB3 antibody and adequate chromatin fragmentation for ChIP (Figure 3A). Subsequent qPCR of immunoprecipitated DNA revealed notable enrichment for specific primer pairs, indicating potential CREB3 binding regions within the MIR210HG promoter (Figure 3B). Additionally, the assessment of chromatin fragmentation confirmed that the size of fragmented DNA ranged from 100 to 500 bp, meeting the requirements for subsequent ChIP enrichment experiments (Figure 3C). ChIP-qPCR results further highlighted the enrichment of six primer sets targeting two distinct regions of MIR210HG, demonstrating significant enrichment in primers corresponding to both target sites (Figure 3D).
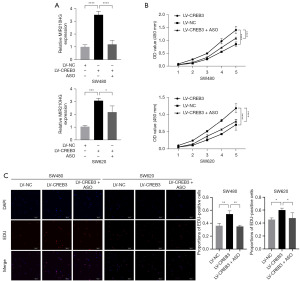
CREB3-mediated transcriptional activation of MIR210HG augments cellular proliferation
A previous study demonstrated the role of MIR210HG in promoting the proliferation of CC cells (21). To investigate the involvement of CREB3 in this process, three distinct sets of cell lines were established: those overexpressing CREB3 alone; those overexpressing CREB3 along with MIR210HG knockdown; and a control group. RT-qPCR analysis confirmed an increase in MIR210HG expression after CREB3 overexpression and a subsequent decrease in expression levels upon MIR210HG knockdown in SW480 and SW620 cells (Figure 4A). Functional assays, including CCK-8 and EdU incorporation assays, demonstrated that CREB3 overexpression significantly enhanced the proliferative capacity of SW480 and SW620 cells. Moreover, the downregulation of MIR210HG partially mitigated the proliferation induced by CREB3 overexpression, as evidenced by both the reduction in absorbance values in the CCK-8 assay (Figure 4B) and the decreased proportion of EdU-positive cells in the EdU assay (Figure 4C). These findings collectively validate the functional significance of CREB3 in the transcriptional upregulation of MIR210HG and its consequent role in promoting cellular proliferation in CC cells.
Discussion
The elucidation of the MIR210HG promoter region and its regulatory mechanisms can provide significant insights into the transcriptional landscape that underlies colon carcinogenesis. The current study delineated the promoter region of MIR210HG and its potential regulation by CREB3, a transcription factor implicated in various cellular processes. The identification of the promoter region, spanning from 2,000 bp upstream to 100 bp downstream of the TSS, is consistent with the recognized architecture of promoters and their role in gene expression regulation. This region location on the antisense strand of chromosome 11 underscores the complexity of transcriptional control, as antisense transcription has been increasingly recognized for its role in diversifying genomic expression and function (22).
The present in silico analysis, leveraging the UCSC Genome Browser and JASPAR database, predicted the CREB3 binding sites within the MIR210HG promoter region. The importance of CREB3 in cellular homeostasis and stress response is well documented (23) and the current findings provide a putative link between CREB3 activity and MIR210HG transcription in CC. The significant co-expression of CREB3 and MIR210HG, as evidenced by starBase v3.0 analysis, further reinforces the potential regulatory interplay between these entities.
The modulation of CREB3 expression in SW480 and SW620 cells, resulting in correlative changes in MIR210HG levels, suggests a regulatory axis that may influence cancer cell phenotype, particularly proliferation. Notably, the CREB3-mediated upregulation of MIR210HG corroborated with increased cellular proliferation, indicating a functional consequence of the activity of this transcription factor. These findings align with existing literature that highlights the role of lncRNAs, such as MIR210HG, in cancer progression through various mechanisms including alterations in cell cycle, apoptosis and metastasis (11-13).
Given these insights, it is essential to consider the potential of MIR210HG as a biomarker for patient risk stratification and local regional metastasis in CC. Elevated levels of MIR210HG expression may correlate with tumor aggressiveness and metastatic potential, suggesting its utility in predicting patient outcomes. Future studies should investigate the relationship between MIR210HG expression levels and clinical parameters such as tumor stage, lymph node involvement, and overall survival rates. Additionally, the feasibility of measuring MIR210HG levels in circulating tumor cells or exosomes could provide a non-invasive method for monitoring disease progression and treatment response, thereby enhancing clinical decision-making.
However, despite the promise of lncRNAs like MIR210HG in clinical applications, several challenges must be addressed. One major challenge is the heterogeneity of lncRNA expression across different cancer types and stages, which complicates their use as reliable biomarkers. Additionally, the lack of standardized protocols for lncRNA detection and quantification hinders their integration into clinical practice. Furthermore, the biological functions of many lncRNAs remain poorly understood, making it difficult to predict their roles in disease progression and therapy response. Addressing these issues will require collaborative efforts in research, including large-scale studies to validate the clinical significance of lncRNAs and the development of robust methodologies for their analysis.
The promoter activity assay performed in this investigation demonstrated that CREB3 can augment the transcriptional activity of the promoter, thereby facilitating the expression of MIR210HG. The ChIP assays provide concrete evidence supporting the direct binding of CREB3 to the MIR210HG promoter region, consolidating the hypothesis of CREB3 being involved in the transcriptional activation of MIR210HG. The significant enrichment of specific primer pairs during qPCR analysis indicates that these regions within the promoter are likely key sites for CREB3 interaction.
The functional implications of CREB3’s transcriptional activation of MIR210HG are substantial, as the current experiments demonstrated that MIR210HG knockdown can attenuate CREB3-induced proliferation in CC cells. This suggests that MIR210HG acts downstream of CREB3 to promote cellular proliferation, a hallmark of cancer progression. The partial rescue of the proliferative phenotype upon MIR210HG knockdown highlights the complexity of the role of CREB3 in oncogenesis, suggesting that while it may be a significant player, it is not the sole contributor to the proliferation of cancer cells.
In conclusion, the current study provides compelling evidence for a CREB3-MIR210HG regulatory axis that contributes to the molecular underpinnings of CC. The delineation of the MIR210HG promoter region, coupled with the validation of CREB3 binding and regulatory impact, enhances the current understanding of how transcription factors orchestrate the expression of non-coding RNAs to influence cancer cell behavior. The implications of these findings extend beyond the realm of basic science, as they may inform future therapeutic strategies targeting the CREB3-MIR210HG interaction in CC treatment. Further research is warranted to elucidate the full spectrum of CREB3 targets and the downstream effects of MIR210HG regulation, potentially paving the way for novel interventions in CC management.
Conclusions
In this study, we elucidated the regulatory mechanism of the CREB3-MIR210HG axis in promoting CC cell proliferation. Our findings demonstrate that CREB3 directly interacts with the MIR210HG promoter, leading to enhanced expression levels of MIR210HG. Functional assays showed that silencing MIR210HG mitigated CREB3-induced proliferation in CC cells, suggesting a critical role for this lncRNA in CC growth. These results highlight the potential of targeting the CREB3-MIR210HG pathway as a novel therapeutic approach in CC treatment. Future investigations are warranted to explore the underlying mechanisms and develop lncRNA-based strategies that could improve therapeutic efficacy in CC patients.
Acknowledgments
We thank the State Key Laboratory of Tumor Biology for providing the molecular biology experimental platform, the National Clinical Research Center for providing the clinical data, and the staff of the Department of Digestive Surgery of Xijing Hospital for their support and assistance in this study.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1525/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1525/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1525/prf
Funding: The present study was funded by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-1525/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kang M, Tang B, Li J, et al. Identification of miPEP133 as a novel tumor-suppressor microprotein encoded by miR-34a pri-miRNA. Mol Cancer 2020;19:143. Erratum in: Mol Cancer 2024;23:195. [Crossref] [PubMed]
- Wang L, Yang J, Wang HN, et al. LncRNA BCYRN1-induced autophagy enhances asparaginase resistance in extranodal NK/T-cell lymphoma. Theranostics 2021;11:925-40. [Crossref] [PubMed]
- Haidara N, Giannini M, Porrua O. Modulated termination of non-coding transcription partakes in the regulation of gene expression. Nucleic Acids Res 2022;50:1430-48. [Crossref] [PubMed]
- Proietti S, Cucina A, Pensotti A, et al. Tumor reversion and embryo morphogenetic factors. Semin Cancer Biol 2022;79:83-90. [Crossref] [PubMed]
- Joshi M, Rajender S. Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod Biol Endocrinol 2020;18:103. [Crossref] [PubMed]
- Kalhori MR, Khodayari H, Khodayari S, et al. Regulation of Long Non-Coding RNAs by Plant Secondary Metabolites: A Novel Anticancer Therapeutic Approach. Cancers (Basel) 2021;13:1274. [Crossref] [PubMed]
- Luo A, Lan X, Qiu Q, et al. LncRNA SFTA1P promotes cervical cancer progression by interaction with PTBP1 to facilitate TPM4 mRNA degradation. Cell Death Dis 2022;13:936. [Crossref] [PubMed]
- Wang H, Shi W, Lu J, et al. HCC: RNA-Sequencing in Cirrhosis. Biomolecules 2023;13:141. [Crossref] [PubMed]
- Fa X, Song P, Fu Y, et al. Long non-coding RNA VPS9D1-AS1 facilitates cell proliferation, migration and stemness in hepatocellular carcinoma. Cancer Cell Int 2021;21:131. [Crossref] [PubMed]
- Feng F, Yang J, Chen A, et al. Long non-coding RNA long intergenic non-protein coding RNA 1232 promotes cell proliferation, migration and invasion in bladder cancer via modulating miR-370-5p/PIM3 axis. J Tissue Eng Regen Med 2022;16:575-85. [Crossref] [PubMed]
- Liu P, Huang H, Qi X, et al. Hypoxia-Induced LncRNA-MIR210HG Promotes Cancer Progression By Inhibiting HIF-1α Degradation in Ovarian Cancer. Front Oncol 2021;11:701488. [Crossref] [PubMed]
- Ma J, Kong FF, Yang D, et al. lncRNA MIR210HG promotes the progression of endometrial cancer by sponging miR-337-3p/137 via the HMGA2-TGF-β/Wnt pathway. Mol Ther Nucleic Acids 2021;24:905-22. [Crossref] [PubMed]
- Shi W, Tang Y, Lu J, et al. MIR210HG promotes breast cancer progression by IGF2BP1 mediated m6A modification. Cell Biosci 2022;12:38. [Crossref] [PubMed]
- Yadav G, Kulshreshtha R. Pan-cancer analyses identify MIR210HG overexpression, epigenetic regulation and oncogenic role in human tumors and its interaction with the tumor microenvironment. Life Sci 2024;339:122438. [Crossref] [PubMed]
- Min W, Dai D, Wang J, et al. Long Noncoding RNA miR210HG as a Potential Biomarker for the Diagnosis of Glioma. PLoS One 2016;11:e0160451. [Crossref] [PubMed]
- Wang AH, Jin CH, Cui GY, et al. MIR210HG promotes cell proliferation and invasion by regulating miR-503-5p/TRAF4 axis in cervical cancer. Aging (Albany NY) 2020;12:3205-17. [Crossref] [PubMed]
- Liu SJ, Dang HX, Lim DA, et al. Long noncoding RNAs in cancer metastasis. Nat Rev Cancer 2021;21:446-60. [Crossref] [PubMed]
- Liu W, Zhang Y, Chen M, et al. A genome-wide analysis of long noncoding RNA profile identifies differentially expressed lncRNAs associated with Esophageal cancer. Cancer Med 2018;7:4181-9. [Crossref] [PubMed]
- Hur K, Kim SH, Kim JM. Potential Implications of Long Noncoding RNAs in Autoimmune Diseases. Immune Netw 2019;19:e4. [Crossref] [PubMed]
- Fernández-Cortés M, Andrés-León E, Oliver FJ. The PARP Inhibitor Olaparib Modulates the Transcriptional Regulatory Networks of Long Non-Coding RNAs during Vasculogenic Mimicry. Cells 2020;9:2690. [Crossref] [PubMed]
- Ruan Z, Xu Z, Li Z, et al. Integral analyses of survival-related long non-coding RNA MIR210HG and its prognostic role in colon cancer. Oncol Lett 2019;18:1107-16. [Crossref] [PubMed]
- Ferrer J, Dimitrova N. Transcription regulation by long non-coding RNAs: mechanisms and disease relevance. Nat Rev Mol Cell Biol 2024;25:396-415. [Crossref] [PubMed]
- Yuxiong W, Faping L, Bin L, et al. Regulatory mechanisms of the cAMP-responsive element binding protein 3 (CREB3) family in cancers. Biomed Pharmacother 2023;166:115335. [Crossref] [PubMed]


