
Construction and validation of a clinical prognostic model for frontal glioblastoma: a real-world clinical study based on radiation therapy
Highlight box
Key findings
• We collected clinical case data of patients with frontal glioblastoma from the open-source tumor database, performed univariate and multivariate analysis to screen out risk factors affecting patient survival and prognosis. The receiver operating characteristic curve, calibration curve and decision curve analysis were used to validate the model, and the Kaplan-Meier method was used for survival analysis.
What is known and what is new?
• Frontal glioblastoma is a common disease in most countries. However, the understanding of the disease is still far from our expectations. Our findings demonstrated that age, laterality, surgery, radiotherapy, chemotherapy and distant metastasis are independent risk factors for patient survival and prognosis. The constructed model provides a good basis for clinical decision-making. Radiation therapy significantly improved the prognosis of patients, which provided a strong basis for clinical treatment and rewriting the expert consensus.
• The innovation of this study is that, for the first time, glioma was statistically analyzed according to the primary site and the survival of patients with frontal lobe development was compared, which provides an important basis for treatment decision-making.
What is the implication, and what should change now?
• These findings will have a significant impact on the broad field of central nervous system tumors. However, current treatment of glioma needs to reflect the sensitivity to temozolomide according to O6-methylguanine-DNA methyltransferase (MGMT) methylation status. Important indicators such as IDH mutation, 1p19q co-deletion, and MGMT promoter methylation status have been included in guidelines and expert consensus.
Introduction
Primary glioma is a tumor of epithelial tissue origin and is the most common malignant tumor in the central nervous system. Characteristics of gliomas include aggressiveness and high malignancy, leading to a very poor prognosis (1). Statistics show that the direct medical costs of primary glioma are 3.2 billion euros per year and many patients discontinue treatment due to its high cost, leading to a poor prognosis, seriously affecting survival time (2). According to Surveillance, Epidemiology, and End Results (SEER) database, glioma is the most common type of malignant brain tumor in the US. Glioblastoma is the most common type of glioma, accounting for about 45% of all gliomas, and its 5-year survival rate is only around 5%, the morbidity and mortality varying by race. Similarly, the disease has some geographical correlation, with the United States, Canada, Australia and Northern Europe having the highest incidence in the world (3,4). Currently, the classic treatment for glioblastoma is surgery combined with preoperative or postoperative radiotherapy and chemotherapy. With the development of chemotherapeutic drugs and the continuous improvement of radiotherapy technology, the survival rate of glioblastoma patients has improved to a certain extent. However, the median survival after diagnosis is only 12 to 14 months (5).
The frontal lobe is located at the forefront of the cerebral hemisphere and regulates voluntary body movements and higher mental activities. Studies have shown that older patients with frontal lobe tumors have a significantly higher rate of cognitive impairment than patients with gliomas at other sites. Frontal lobe tissue delays neurodegeneration and cognitive decline (6). During the clinical consultation, some patients have a headache and epilepsy as the main clinical manifestations and are prone to delirium after surgery. After studying these cases, it was found that most of them were frontal gliomas, which also suggests frontal lobe tumors’ hazards and concealment (7). The higher mental activities dominated by the left and right frontal lobes are not all the same. The left frontal lobe has a greater impact on executive function and language ability (6,8-10), whereas the right frontal lobe has a greater role in regulating attention and functional recovery. Therefore, in the process of surgical resection, neurosurgeons should not only aim to remove the tumor, but also pay attention to protecting the normal frontal lobe tissue from damage, which also highlights the importance of the frontal lobe.
Therefore, the construction of survival and prognosis models for frontal glioblastoma is crucial for the selection of clinical treatments and there are few reports in the existing literature on the above direction. The aim of this study is to identify risk factors for frontal glioblastoma, to construct survival models, and to provide strong evidence for patients and doctors to apply radiotherapy to frontal glioblastoma. We would like to provide strong data support for clinical diagnosis and treatment. We present this article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2058/rc).
Methods
Research objects and data collection
Patients with pathologically confirmed glioblastoma with complete medical records between 2004 and 2015 were retrieved from the SEER database using SEER*Stat 8.4.0 software (ssp://seerinsweb.com:2038). SEER database is an open-access database. All data are reserved. The selected cases were based on the site code and histological code defined by the third edition of the International Classification of Diseases for Oncology (ICD-O-3). Histological types included in this study include glioblastoma, giant cell glioblastoma, gliosarcoma. Patients were considered invalid if primary data (including age, sex, surgery, laterality, race, marital status, chemoradiotherapy information, survival time, and status) were missing. A total of 2,063 patients with glioblastoma in the frontal lobe were included in this study. They were divided into a training cohort and a validation cohort by the random number function method according to the ratio of 7:3, with 1,444 cases in the training cohort and 619 cases in the validation cohort. The data flowchart is shown in Figure 1. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Research variables
Description and statistics of patients with frontal glioblastoma according to the following variables: basic patient characteristics (age, gender, marital status, race), disease characteristics (World Health Organization classification, transversality, metastasis) and treatment options (surgery, radiation, chemotherapy).
Selection of cut-off value
When performing data statistics and model building, it is necessary to convert the continuous variables into categorical variables, and to use Kaplan-Meier method in X-tile software to select and determine the cut-off value. The cut-off value is selected after a variety of statistical methods validation.
Cox univariate and multivariate analysis and model construction
Univariate and multivariate Cox regression analysis was used to determine the overall survival (OS), cancer-specific survival (CSS) and independent prognostic factors in the training cohort. Training cohorts for OS, CSS time were constructed by combining patients’ basic characteristics (age, sex, marital status, race), disease characteristics, and treatment. A queue of states that expresses the mathematical model through a nomogram. The authenticity and reliability of the nomogram model are evaluated by the concordance index (C-index). The closer the C-index is to 1, the stronger the discriminatory power is.
When C-index is below 0.5, the model is considered a failure. The agreement between predicted and observed values in training and validation cohorts was assessed by calibration curves. The above steps are done by R 4.0.4.
Validation of model
Rms package, foreign package and survival package in R 4.0.4 were used to calculate the consistency index of the prognostic model. If the consistency index was greater than 0.7, the model could be considered to be highly reliable. The receiver operating characteristic (ROC) curve of 1-, 3-, and 5-year OS and CSS were plotted using the SurvivalROC package in R 4.0.4. The predictive value of the model increased as the area under the curves approached 1. The Bootstrap method was used to construct the calibration curve. The higher the degree of coincidence between the broken line and the standard line, the higher the degree of credibility of the model. Decision curve analysis (DCA) package was used to draw the clinical decision curve and evaluate the clinical prediction efficiency of the model.
Statistical analysis
Population survival analyses were performed using log-rank (Mantel-Cox) testing. The survival curve was achieved by Graphpad software, and the HR, P value and 95% confidence interval (CI) were reported. The test level was set at α=0.05, and P<0.05 was considered statistically significant. In order to balance baseline differences between groups, R 4.0.4 was used to perform propensity score matching (PSM), and patients were divided into radiotherapy group and non-radiotherapy group according to whether they received radiotherapy at ratio of 1:1, with a caliper value of 0.02. The matched cases information was collected completely.
Data and image processing
The random number function of Excel 2016 was used to group the data, and the rms package and survival package of R 4.0.4 were used for univariate and multivariate Cox regressions and survival analysis. The pictures were tackled by Adobe Photograph 2020. Survival analysis was finished by Graphpad 7.0.
Results
Patient clinical features
A total of 2,063 patients were included in this study, including 1,150 males (55.74%) and 913 females (44.26%); 903 patients aged below 60 years old, 713 patients aged 60–72 years old, and 447 patients aged 73–93 years old; 1,666 cases (80.76%) received radiotherapy, 1,513 cases (73.34%) received chemotherapy, and 1,828 (88.61%) received surgical treatments. According to the World Health Organization (WHO) central nervous system (CNS) tumor classification, they were divided into grades I–IV, of which 1,910 cases were grade IV (Table 1).
Table 1
| Variable | All (n=2,063), n (%) | Training cohort (n=1,444), n (%) | Validation cohort (n=619), n (%) |
|---|---|---|---|
| Age at diagnosis | |||
| <60 years | 903 (43.77) | 626 (43.35) | 277 (44.75) |
| 60–72 years | 713 (34.56) | 506 (35.04) | 207 (33.44) |
| >72 years | 447 (21.67) | 312 (21.61) | 135 (21.81) |
| Gender | |||
| Female | 913 (44.26) | 646 (44.74) | 267 (43.13) |
| Male | 1,150 (55.74) | 798 (55.26) | 352 (56.87) |
| Marriage status | |||
| Single | 630 (30.54) | 440 (30.47) | 190 (30.69) |
| Married | 1,373 (66.55) | 963 (66.69) | 410 (66.24) |
| Unknown | 60 (2.91) | 41 (2.84) | 19 (3.07) |
| Race | |||
| Black | 110 (5.33) | 80 (5.54) | 30 (4.85) |
| White | 1,864 (90.35) | 1,311 (90.79) | 553 (89.34) |
| Other | 89 (4.31) | 53 (3.67) | 36 (5.82) |
| Grade | |||
| WHO I | 13 (0.63) | 9 (0.62) | 4 (0.65) |
| WHO II | 8 (0.39) | 4 (0.28) | 4 (0.65) |
| WHO III | 132 (6.40) | 93 (6.44) | 39 (6.30) |
| WHO IV | 1,910 (92.60) | 1,338 (92.66) | 572 (92.41) |
| Laterality | |||
| Left | 943 (45.71) | 642 (44.46) | 301 (48.63) |
| Right | 1,058 (51.28) | 759 (52.56) | 299 (48.30) |
| Bilateral | 62 (3.01) | 43 (2.98) | 19 (3.07) |
| Distant metastasis | |||
| Regional | 490 (23.75) | 338 (23.41) | 152 (24.56) |
| Localized | 1,537 (74.50) | 1,082 (74.93) | 455 (73.51) |
| Distant | 36 (1.75) | 24 (1.66) | 12 (1.94) |
| Surgery | |||
| No/unknown | 235 (11.39) | 169 (11.70) | 66 (10.66) |
| Yes | 1,828 (88.61) | 1,275 (88.30) | 553 (89.34) |
| Radiotherapy | |||
| No/unknown | 397 (19.24) | 274 (18.98) | 123 (19.87) |
| Yes | 1,666 (80.76) | 1,170 (81.02) | 496 (80.13) |
| Chemotherapy | |||
| No/unknown | 550 (26.66) | 378 (26.18) | 172 (27.79) |
| Yes | 1,513 (73.34) | 1,066 (73.82) | 447 (72.21) |
WHO, World Health Organization.
Determination of cut-off values for continuous variables
The age variable included in this study was a continuous variable. Kaplan-Meier method was used to perform univariate survival analysis and to select the best cut-off value. The age group variables were defined as “below 60 years”, “60–72 years” and “73–93 years”, and the age variables were specified to convert continuous variables into categorical variables.
Training and validation cohort data
A total of 2,063 cases with complete case information were included in this study. According to the principle of medical statistics optimization, the cases were divided into a training group and a verification group by 7:3 using a random number function. A total of 1,444 cases were included in the training cohort and a total of 619 cases were included in the validation group. The cases in the training cohort were used to build the model and the data in the validation cohort was used for model validation.
Univariate and multivariate Cox regression and nomogram constructed
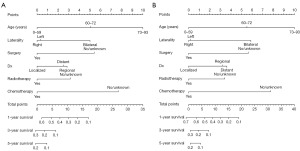
Univariate Cox regression analysis using the rms package in R 4.0.4 showed that age, laterality, distant metastasis, whether to perform surgery, radiotherapy or chemotherapy were independent risk factors for the prognosis of patients with glioblastoma. The differences were shown in Tables 2,3. The above variables were included in the multivariate Cox regression analysis to construct an independent risk model for predicting prognosis, and the multivariate Cox regression model was plotted by R 4.0.4 to produce a nomogram of prognosis for patients with glioblastoma (Figure 2A,2B).
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||
| Age, years | |||||||
| <60 | Ref. | 1 | Ref. | 1 | |||
| 60–72 | <0.001 | 1.63 | 1.444–1.841 | <0.001 | 1.635 | 1.447–1.848 | |
| 73–93 | <0.001 | 2.974 | 2.579–3.428 | <0.001 | 2.646 | 2.289–3.059 | |
| Gender | |||||||
| Female | Ref. | 1 | Ref. | 1 | |||
| Male | 0.36 | 1.051 | 0.945–1.169 | Ref. | 1 | ||
| Marriage | |||||||
| Single | Ref. | 1 | Ref. | 1 | |||
| Married | 0.32 | 1.061 | 0.944–1.192 | Ref. | 1 | ||
| Unknown | 0.52 | 1.118 | 0.799–1.566 | Ref. | 1 | ||
| Race | |||||||
| Black | Ref. | 1 | Ref. | 1 | |||
| White | 0.18 | 1.174 | 0.929–1.484 | Ref. | 1 | ||
| Other | 0.18 | 0.778 | 0.538–1.124 | Ref. | 1 | ||
| Grade | |||||||
| WHO I | Ref. | 1 | Ref. | 1 | |||
| WHO II | 0.99 | 1.004 | 0.302–3.337 | Ref. | 1 | ||
| WHO III | 0.1 | 1.827 | 0.887–3.765 | Ref. | 1 | ||
| WHO IV | 0.17 | 1.635 | 0.816–3.278 | Ref. | 1 | ||
| Laterality | |||||||
| Left | Ref. | 1 | Ref. | 1 | |||
| Right | 0.56 | 0.969 | 0.870–1.079 | 0.567 | 0.969 | 0.869–1.080 | |
| Bilateral | <0.001 | 1.686 | 1.237–2.299 | <0.001 | 1.576 | 1.149–2.163 | |
| Distant metastasis | |||||||
| Regional | Ref. | 1 | Ref. | 1 | |||
| Localized | <0.001 | 0.746 | 0.658–0.845 | <0.001 | 0.759 | 0.667–0.863 | |
| Distant | 0.7 | 1.085 | 0.716–1.642 | 0.87 | 0.965 | 0.636–1.465 | |
| Surgery | |||||||
| No/unknown | Ref. | 1 | Ref. | 1 | |||
| Yes | <0.001 | 0.565 | 0.481–0.665 | <0.001 | 0.591 | 0.498–0.701 | |
| Radiotherapy | |||||||
| No/unknown | Ref. | 1 | Ref. | 1 | |||
| Yes | <0.001 | 0.449 | 0.392–0.514 | <0.001 | 0.735 | 0.614–0.880 | |
| Chemotherapy | |||||||
| No/unknown | Ref. | 1 | Ref. | 1 | |||
| Yes | <0.001 | 0.385 | 0.341–0.435 | <0.001 | 0.474 | 0.404–0.556 | |
CI, confidence interval; HR, hazard ratio; WHO, World Health Organization.
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||
| Age, years | |||||||
| <60 | Ref. | 1 | Ref. | 1 | |||
| 60–72 | <0.001 | 1.662 | 1.464–1.889 | <0.001 | 1.674 | 1.472–1.904 | |
| 73–93 | <0.001 | 2.859 | 2.456–3.329 | <0.001 | 2.548 | 2.182–2.974 | |
| Gender | |||||||
| Female | Ref. | 1 | Ref. | 1 | |||
| Male | 0.31 | 1.06 | 0.947–1.186 | Ref. | 1 | ||
| Marriage | |||||||
| Single | Ref. | 1 | Ref. | 1 | |||
| Married | 0.15 | 1.094 | 0.967–1.237 | Ref. | 1 | ||
| Unknown | 0.96 | 0.991 | 0.679–1.446 | Ref. | 1 | ||
| Race | |||||||
| Black | Ref. | 1 | Ref. | 1 | |||
| White | 0.09 | 1.242 | 0.964–1.601 | Ref. | 1 | ||
| Other | 0.25 | 0.793 | 0.534–1.179 | Ref. | 1 | ||
| Grade | |||||||
| WHO I | Ref. | 1 | Ref. | 1 | |||
| WHO II | 0.1 | 1.002 | 0.302–3.328 | Ref. | 1 | ||
| WHO III | 0.19 | 1.627 | 0.787–3.365 | Ref. | 1 | ||
| WHO IV | 0.28 | 1.465 | 0.730–2.937 | Ref. | 1 | ||
| Laterality | |||||||
| Left | Ref. | 1 | Ref. | 1 | |||
| Right | 0.56 | 0.969 | 0.870–1.079 | 0.75 | 0.982 | 0.875–1.101 | |
| Bilateral | <0.001 | 1.686 | 1.237–2.299 | <0.001 | 1.701 | 1.229–2.355 | |
| Distant metastasis | |||||||
| Regional | Ref. | 1 | Ref. | 1 | |||
| Localized | <0.001 | 0.725 | 0.636–0.826 | <0.001 | 0.741 | 0.647–0.848 | |
| Distant | 0.52 | 1.15 | 0.752–1.758 | 0.86 | 1.038 | 0.678–1.591 | |
| Surgery | |||||||
| No/unknown | Ref. | 1 | Ref. | 1 | |||
| Yes | <0.001 | 0.561 | 0.472–0.666 | <0.001 | 0.59 | 0.493–0.706 | |
| Radiotherapy | |||||||
| No/unknown | Ref. | 1 | Ref. | 1 | |||
| Yes | <0.001 | 0.455 | 0.394–0.526 | <0.001 | 0.726 | 0.600–0.880 | |
| Chemotherapy | |||||||
| No/unknown | Ref. | 1 | Ref. | 1 | |||
| Yes | <0.001 | 0.393 | 0.345–0.448 | <0.001 | 0.485 | 0.409–0.575 | |
CI, confidence interval; HR, hazard ratio; WHO, World Health Organization.
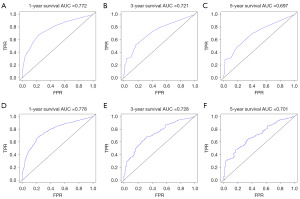
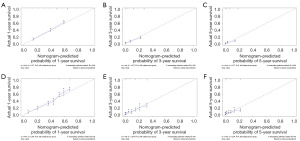
Model validation and evaluation results
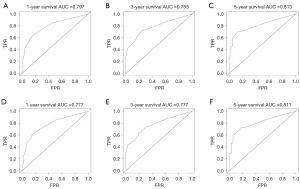
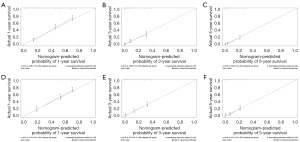
The constructed model was evaluated using R 4.0.4 and by calculating the C-index, it can be seen that the model has good reliability, C-index =0.712 (standard error =0.007). The area under the ROC curve (AUC) was used for validation. As shown in Figure 3A-3F, the AUC of 1-year OS and CSS prediction model of glioblastoma cells was 0.772 and 0.778, of 3-year OS and CSS prediction model was 0.721 and 0.728, and of 5-year OS and CSS prediction model was 0.697 and 0.701. The calibration curve model using R language was well fitted with good predictive value for 1-, 3- and 5-year survival prediction models for patients with glioblastoma (Figure 4A-4F).
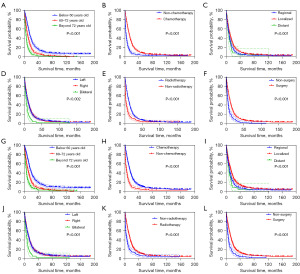
Survival analysis results
Survival analysis was performed for age variables, laterality variables, surgical variables, radiotherapy variables, chemotherapy variables, and distant metastasis variables. Survival analysis was performed using the Kaplan-Meier method, and P<0.05 was considered statistically significant. The prognosis of patients with glioblastoma was closely related, and the difference was statistically significant (Figure 5).
Validation cohort data to validate the model
The ROC curve results showed that the AUC of 1-year OS and CSS model was 0.797 and 0.777; the 3-year survival model was 0.785 and 0.777, and the 5-year survival model was 0.813 and 0.811 (Figure 6A-6F). The calibration curves showed that the 1-, 3-, and 5-year approached the midline level, and the model was constructed with a high degree of reliability (Figure 7A-7F).
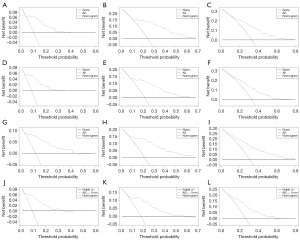
Clinical decision curve and evaluation on clinical prediction efficiency of the model
The DCA curve proves that the modified tumor node metastasis (TNM) stage model has better clinical predictive efficacy for the individualized diagnosis and treatment of frontal glioblastoma patients, and it can be applied in clinical work (Figure 8A-8L). The more the curve on the right deviates from the diagonal line on the left, the better the clinical prediction efficiency of the model and the more conducive to clinical decision-making.
PSM was performed according to the situation of radiotherapy
According to whether radiotherapy was performed or not, 2,063 cases were grouped and matched according to a 1:1 ratio, and the caliper value was 0.02. A total of 450 cases were screened out. In the survival analysis of these cases, the median survival was 7 months for those who received radiation therapy and 5 months for those who did not receive radiation therapy [hazard ratio (HR) =1.067, P=0.01; Figure 9, Tables 4,5].
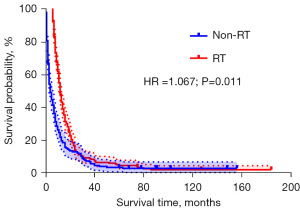
Table 4
| Variables | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| RT (n=1,666) | Non-RT (n=397) | SMD | RT (n=225) | Non-RT (n=225) | SMD | ||
| Age, n (%) | |||||||
| <60 years | 776 (46.6) | 127 (32.0) | −0.313 | 76 (33.8) | 103 (45.8) | 0.257 | |
| 60–72 years | 586 (35.2) | 127 (32.0) | −0.068 | 78 (34.7) | 51 (22.7) | −0.257 | |
| >72 years | 304 (18.2) | 143 (36.0) | 0.370 | 71 (31.6) | 71 (31.6) | 0.000 | |
| Laterality, n (%) | |||||||
| Left | 761 (45.7) | 182 (45.8) | 0.003 | 94 (41.8) | 94 (41.8) | 0.000 | |
| Right | 859 (51.6) | 199 (50.1) | −0.029 | 129 (57.3) | 129 (57.3) | 0.000 | |
| Bilateral | 46 (2.8) | 16 (4.0) | 0.065 | 2 (0.9) | 2 (0.9) | 0.000 | |
| Distant metastasis, n (%) | |||||||
| Regional | 387 (23.2) | 103 (25.9) | 0.062 | 39 (17.3) | 39 (17.3) | 0.000 | |
| Localized | 1,254 (75.3) | 283 (71.3) | −0.088 | 180 (80.0) | 180 (80.0) | 0.000 | |
| Distant | 25 (1.5) | 11 (2.8) | 0.077 | 6 (2.7) | 6 (2.7) | 0.000 | |
| Surgery, n (%) | |||||||
| No/unknown | 227 (13.6) | 8 (2.0) | −0.826 | 8 (3.6) | 8 (3.6) | 0.000 | |
| Yes | 1,439 (86.4) | 389 (98.0) | 0.826 | 217 (96.4) | 217 (96.4) | 0.000 | |
| Chemotherapy, n (%) | |||||||
| No/unknown | 209 (12.5) | 341 (85.9) | 2.107 | 169 (75.1) | 169 (75.1) | 0.000 | |
| Yes | 1457 (87.5) | 56 (14.1) | −2.107 | 56 (24.9) | 56 (24.9) | 0.000 | |
RT, radiotherapy; SMD, standardized mean difference.
Table 5
| Characteristics | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| RT (N=1,666) | Non-RT (N=397) | P | RT (N=225) | Non-RT (N=225) | P | ||
| Age, n (%) | <0.001 | 0.008 | |||||
| <60 years | 776 (46.6) | 127 (32.0) | 76 (33.8) | 103 (45.8) | |||
| 60–72 years | 586 (35.2) | 127 (32.0) | 78 (34.7) | 51 (22.7) | |||
| >72 years | 304 (18.2) | 143 (36.0) | 71 (31.6) | 71 (31.6) | |||
| Laterality, n (%) | 0.38 | >0.99 | |||||
| Left | 761 (45.7) | 182 (45.8) | 94 (41.8) | 94 (41.8) | |||
| Right | 859 (51.6) | 199 (50.1) | 129 (57.3) | 129 (57.3) | |||
| Bilateral | 46 (2.8) | 16 (4.0) | 2 (0.9) | 2 (0.9) | |||
| Distant metastasis, n (%) | 0.10 | >0.99 | |||||
| Regional | 387 (23.2) | 103 (25.9) | 39 (17.3) | 39 (17.3) | |||
| Localized | 1,254 (75.3) | 283 (71.3) | 180 (80) | 180 (80) | |||
| Distant | 25 (1.5) | 11 (2.8) | 6 (2.7) | 6 (2.7) | |||
| Surgery, n (%) | <0.001 | >0.99 | |||||
| No/unknown | 227 (13.6) | 8 (2.0) | 8 (3.6) | 8 (3.6) | |||
| Yes | 1,439 (86.4) | 389 (98.0) | 217 (96.4) | 217 (96.4) | |||
| Chemotherapy, n (%) | <0.001 | >0.99 | |||||
| No/unknown | 209 (12.5) | 341 (85.9) | 169 (75.1) | 169 (75.1) | |||
| Yes | 1,457 (87.5) | 56 (14.1) | 56 (24.9) | 56 (24.9) | |||
PSM, propensity score matching; RT, radiotherapy.
Discussion
Glioma is the most common primary brain and central nervous system tumor, which originates from glial stem or progenitor cells (11), with an incidence rate of (4.67–5.73)/100,000 person-years, and 81% of malignant brain tumors are glioma. While glioblastoma accounts for nearly 50% of gliomas, its 5-year survival rate is only 0.05–4.7% (1,4). The latest data show a glioblastoma incidence of about 3.23 per 100,000 people, with a 5-year survival rate of 6.9% (12). According to the World Health Organization classification, Grades I–II are low-grade and Grades III–IV are high-grade. High-grade gliomas are difficult to treat, prone to recurrence, and have an extremely poor prognosis. Without intervention, the tumor has a tendency to convert to higher grades, and its prognosis is negatively correlated with an increase in grade (5,13). The cancer-specific mortality of glioma is of greater concern than that of tumors with higher incidence, such as lung cancer and breast cancer. However, as a common malignant tumor of the central nervous system, gliomas progress slowly, and patient survival is severely compromised. Glioblastoma has a very high degree of malignancy. As a subtype of glioma, it has the characteristics of shorter survival and easier metastasis than other types (14-18).
Frontal lobe tumors tend to impair cognitive abilities, while mild cognitive impairment is difficult to detect and patients do not choose to seek medical attention until they present with epileptic symptoms (6). Dorsolateral prefrontal lobe tumors predispose to executive dysfunction and decreased decision-making ability, usually characterized by prolonged executive response time and inattention (6,19). There have been reports of undetected cognitive, anterograde amnesia, fictitiousness and obsessive-compulsive symptoms (20,21).
In the present study, the C-index value of the training group nomogram for OS and CSS of primary glioma constructed by Xia was 0.688. The reliability of the model has been validated as good value. In the survival model for glioblastoma patients constructed in this study, the C-index value of OS and CSS in the training group was 0.712 and 0.710, and the C-index value of OS and CSS in the validation group was 0.797 and 0.777, both of which had a high reliability value. It is an important reference value for diagnosis and treatment (5).
In this study, patients with pathologically confirmed glioblastoma, a common subtype of glioma in the WHO classification, who had complete case information between 2004 and 2015 were included. Plasmoblastomas can be subdivided into many subtypes, and the cases included in this study contained patients with gliosarcoma, which is considered a subtype of isocitrate dehydrogenase-1 (IDH1) wild-type glioblastoma. The same treatment plan was used as for glioma. The classical treatment options include surgery, radiation therapy and chemotherapy, with temozolomide as the main chemotherapy (22). In the case screening, patients with gliosarcoma were not excluded to avoid selection bias, but its inclusion in the glioma cohort may not be justified due to its sarcoma component, as it is considered a specific subtype of glioblastoma. We believe that its inclusion in the study is more scientific.
The incidence and survival patterns of the vast majority of gliomas have been reported to vary by race, with non-Hispanic whites having higher incidence and shorter survival compared to other populations. However, in the survival prognosis model constructed in this study, we did not observe this phenomenon, and there were no statistical differences in the survival prognosis of patients with different races. The non-significance in race might be due to the selection bias caused by the completion of data, or due to patients’ short survival time caused by high malignancy and poor prognosis. To validate the association between race and prognosis, additional cases need to be included and evaluated with the same inclusion criteria (3).
Due to the existence of blood-brain barrier, many chemotherapeutic drugs are ineffective for glioma, so radiotherapy has become an important means of treatment. Based on this study, we found that local treatment intervention significantly improved OS. With the continuous development of stereotactic radiotherapy technology and image-guided technology, higher radiation doses can be achieved inside the tumor, which overcomes the problem that chemotherapy drugs cannot pass the blood-brain barrier.
However, there are certain limitations in this study. Current treatment of glioma needs to reflect the sensitivity to temozolomide according to O6-methylguanine-DNA methyltransferase (MGMT) methylation status. Important indicators such as IDH mutation, 1p19q co-deletion, and MGMT promoter methylation status have been included in guidelines and expert consensus. The molecular biological indicators mentioned above have not been included in the SEER database. We will do our best to fill this gap in our future practice of establishing our center and national tumor database. The population included in the information includes oncology patients of all races and regions from around the world. The reliance on the SEER database limits generalizability to non-American populations.
Conclusions
The nomogram model constructed in this study has high clinical efficacy, and it can provide a strong reference to develop individualized clinical treatment plans for patients. As an important therapeutic method, radiotherapy can be recommended in the treatment of glioblastoma, which can improve the local control rate and OS of patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2058/rc
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2058/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-24-2058/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li XY, Shang XJ, Xia T, et al. Risk factors for prognosis of patients with primary brain glioma:an analysis based on the SEER database. Chinese Journal of Clinical Neurosurgery 2021;26:764-8.
- Deng Z, Li X, Yang J, et al. Marital Status Independently Predicts Glioma Patient Mortality: A Surveillance, Epidemiology, and End Results (SEER) Analysis. World Neurosurg 2021;152:e302-12. [Crossref] [PubMed]
- Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 2014;16:896-913. [Crossref] [PubMed]
- Morgan LL. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 2015;17:623-4. [Crossref] [PubMed]
- Xia Y, Liao W, Huang S, et al. Nomograms for Predicting the Overall and Cancer-Specific Survival of Patients with High-Grade Glioma: A Surveillance, Epidemiology, and End Results Study. Turk Neurosurg 2020;30:48-59. [PubMed]
- Li BH. Effects of frontal lobe tumors and surgical intervention on advanced cognitive function in the elderly. Journal of International Neurology and Neurosurgery 2018;45:304-7.
- Jia Y, Zhou YP, Wang ZG. Analysis and prediction of the influence factors of delirium after operation in adult patients with gliomas. Chinese Journal of Cancer Prevention and Treatment 2021;28:1477-81.
- Yu G, Bao WM, Mao Y, et al. Study of Executive Functions of Patients with Tumor in Both Right and Left Frontal Lobe. Chinese Journal of Clinical Neurosciences 2010;18:54-7.
- Zhang YL, Huang FE, Miao DM, et al. Left hemisphere tumor affects sequential-verbal abilities. Journal of the Fourth Military Medical University 2002;23:997-9.
- Guo HJ, Fu XM, Niu CS, et al. Study of attentional function of patients with tumor in right and left frontal lobe. Chinese Journal of Stereotactic and Functional Neurosurgery 2008;21:81-4.
- Liu H, Qin X, Zhao L, et al. Epidemiology and Survival of Patients With Brainstem Gliomas: A Population-Based Study Using the SEER Database. Front Oncol 2021;11:692097. [Crossref] [PubMed]
- Rodríguez-Mendoza B, Figueroa-González A, Cano-Herrera G, et al. Glioblastoma and its interaction with neurogenesis. Rev Neurol 2024;79:279-87. [Crossref] [PubMed]
- Yang Y, Yao M, Long S, et al. Prognostic Nomograms for Primary High-Grade Glioma Patients in Adult: A Retrospective Study Based on the SEER Database. Biomed Res Int 2020;2020:1346340. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Li J, Huang W, Chen J, et al. Nomograms for predicting the overall survival of patients with cerebellar glioma: an analysis of the surveillance epidemiology and end results (SEER) database. Sci Rep 2021;11:19348. [Crossref] [PubMed]
- Muskens IS, Feng Q, Francis SS, et al. Pediatric glioma and medulloblastoma risk and population demographics: a Poisson regression analysis. Neurooncol Adv 2020;2:vdaa089. [Crossref] [PubMed]
- Jia Z, Yan Y, Wang J, et al. Development and validation of prognostic nomogram in ependymoma: A retrospective analysis of the SEER database. Cancer Med 2021;10:6140-8. [Crossref] [PubMed]
- Huang Q, Li F, Chen Y, et al. Prognostic factors and clinical outcomes in adult primary gliosarcoma patients: a Surveillance, Epidemiology, and End Results (SEER) analysis from 2004 to 2015. Br J Neurosurg 2020;34:161-7. [Crossref] [PubMed]
- Wang YY, Wan JH, Wang L, et al. Decision-making research in patients of prefrontal lobe tumor. Acta Universitatis Medicinalis Anhui 2016;51:558-60.
- Nazlı ŞB, Sevindik M. Letter to the Editor: Depression As The First Symptom Of Frontal Lobe Grade 2 Malignant Glioma. Turk Psikiyatri Derg 2022;33:143-5.
- Liu YS, Mao ZQ. A case of obsessive-compulsive state caused by right frontal lobe tumor. Chinese Journal of Psychiatry 2008;41:186.
- Alattar AA, Brandel MG, Hirshman BR, et al. Oligodendroglioma resection: a Surveillance, Epidemiology, and End Results (SEER) analysis. J Neurosurg 2018;128:1076-83. [Crossref] [PubMed]



