A transcriptional-microRNA network for β-catenin-driven stemness in hepatocellular carcinoma
Introduction
Wnt/β-catenin pathway is important in maintaining stemness of embryonic, adult and cancer stem cells (CSCs) in various organs including liver. However, the mechanisms underlying its deregulation in liver CSCs are poorly understood. Ma and colleagues have recently demonstrated that Oct4/microRNA-1246 signaling axis activates Wnt/β-catenin signaling in a subset of liver CSCs, providing a mechanistic basis for future diagnostic, prognostic and therapeutic developments.
The cancer stem cells (CSCs) are posited to be responsible not only for tumor initiation but also for tumor relapse and metastasis due to their capacities of self-renewal, drug resistance, and phenotypic reversibility (1). Since the Wnt/β-catenin signaling plays key roles in both adult liver progenitors and CSCs for the regulation of liver embryogenesis, regeneration, and carcinogenesis, detailed characterization of its regulatory mechanisms in CSCs is therefore imperative for improving early detection, prevention and treatment approaches for hepatocellular carcinoma (HCC) (2,3). In this regard, a recent study by Ma and colleagues (4) has revealed a transcriptional-microRNA (miRNA)-β-catenin axis that can induce “stemness” of HCC for self-renewal, tumorigenicity, metastasis and chemoresistance.
Diverse regulation of Wnt/β-catenin signaling in liver cancer
Around half of all HCC patients have activation of the Wnt/β-catenin pathway as a result of gene mutations, epigenetic modifications, or other means. While the frequencies of CTNNB1 and AXIN gene mutations are relatively low (5–20%) in human HCCs, accumulating evidence has underscored the importance of epigenetic deregulation of Wnt/β-catenin signaling via DNA methylation and histone modifications (5). For examples, histone modifiers such as enhancer zeste homolog 2 (EZH2) and histone deacetylase 8 (HDAC8) are frequently over-expressed in human HCCs and contribute to constitutive β-catenin activation via epigenetic silencing of Wnt antagonists (AXIN2, NKD1, PPP2R2B and PRICKLE1) (6,7). Recent studies also unveiled a cell cycle-related kinase (CCRK)/GSK-3β kinase cascade in promoting β-catenin-driven hepatocarcinogenesis (8-10). It is therefore conceivable to anticipate multifaceted regulation of Wnt/β-catenin signaling in liver CSCs.
Transcriptional-miRNA control of Wnt/β-catenin in liver cancer stemness
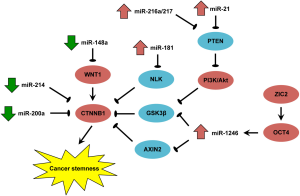
Through miRNA profiling in a CD133+ liver CSC subset with β-catenin activation, Ma and colleagues identified a human miRNA, miR-1246 that specifically suppresses the expression of AXIN2 and GSK-3β, two members of the β-catenin destruction complex, leading to nuclear accumulation and activation of β-catenin (4). Using a constitutively active β-catenin construct (∆45β-cat) that is resistant to phosphorylation-mediated ubiquitination, the investigators demonstrated that β-catenin activation mediates miR-1246-induced tumorigenicity, metastasis and stemness of CD133+ liver CSCs. Clinically, endogenous and secretory miR-1246 over-expression in HCC clinical samples was found to be tightly associated with poor patient survival rates, suggesting its potential application as diagnostic and prognostic biomarker for HCC. Through siRNA-mediated knockdown coupled with chromatin immunoprecipitation and gene expression analysis, another important self-renewal molecule overexpressed in CD133+ liver CSCs, Oct4, was shown to directly up-regulate miR-1246 expression in HCC cells, thus highlighting an Oct4/miR-1246 signaling axis that drives Wnt/β-catenin activation in HCC. These data nicely uncover a new layer of non-genetic mechanism by which the Wnt-mediated stem cell-like properties of HCC cells are induced and sustained, providing important insights for future diagnostic, prognostic and therapeutic developments. Perhaps a missing link that warrants investigation is whether Oct4 could induce Wnt/β-catenin activation by miR-1246, the result of which might further strengthen the functional significance of this miRNA in connecting these two crucial self-renewal pathways (11). Besides, a recent study has shown that the transcription factor ZIC2 is highly expressed in CD13+CD133+ liver CSC subset and initiates Oct4 activation (12), thus suggesting a transcriptional-miRNA cascade for β-catenin-driven stemness in HCC.
MiRNA-mediated regulation of cancer stemness in HCC
MiRNAs are an important class of posttranscriptional regulators and have been demonstrated to be involved in a variety of cellular processes, such as cell proliferation, differentiation and apoptosis. Emerging evidence supports that miRNAs actively participate in the regulation of stemness in human cancer of different tissue origins (13). The deregulation of miRNAs in liver CSCs was first reported by Ji et al. in 2009. Through microarray-based miRNA profiling, the authors demonstrated that miR-181 family members were overexpressed in epithelial cell adhesion molecule (EpCAM)+/α-fetoprotein (AFP)+ liver cancer cells that exhibited stem cell features, including self-renewal and ability to form aggressive tumors in vivo. Importantly, inhibition of miR-181 reduced the number of EpCAM+ HCC cells and tumor-initiating ability, whereas exogenous miR-181 exerted opposite effects. Mechanistically, miR-181 was found to target CDX2 and GATA6 (two important hepatic transcriptional regulators of differentiation) as well as NLK (an inhibitor of Wnt/β-catenin signaling) (14). Using a similar approach, Ma et al. demonstrated that overexpression of miR-130b promoted stemness in CD133+ liver CSCs through targeting TP53INP1 (a positive regulator of p53 signaling) (15). Since then, the number of stemness-regulating miRNAs identified in HCC has continued to grow (Table 1) (16-32). Interestingly, many of the identified miRNAs were found to regulate cancer stemness via direct or indirect modulation of Wnt/β-catenin signaling as illustrated in Figure 1. The current paper by Ma and colleagues further discovered that miR-1246 connects Oct4 and Wnt/β-catenin signaling in the regulation of cancer stemness in HCC (4).

Full table
Future perspectives
As master control of gene expression, miRNAs themselves exhibit differential expression in liver CSCs. These deregulated miRNAs modulate stemness of HCC cells through extensive interaction with intracellular signaling pathways, including the Wnt/β-catenin signaling. Further delineation of the upstream and downstream mechanisms of miRNA deregulation with the state-of-the-art sequencing technologies, such as single-cell epigenomics (33) and transcriptomics (34), will help us better understand CSC biology in HCC and discover novel molecular targets for the development of stem cell-specific therapeutics. In particular, it is tempting to investigate if the distinct miRNA expression profile could give rise to synthetic lethality in liver CSCs. To this end, inhibiting upregulated miRNAs in a systematic manner will help elucidate if targeting a particular miRNA can exclusively induce cell death in HCC stem cells while sparing normal cells. As potential non-invasive biomarkers, it will be interesting to determine if circulating miRNAs could serve as surrogates for tissue stem cell markers for stratifying HCC patients with different clinical outcomes. With these in mind, it is hopeful that stemness-related miRNAs will achieve clinical utilities in the management of HCC in the near future.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (373492), Shenzhen Science and Technology Programme (JCYC20140905151710921), CUHK-Focused Innovations Scheme-Scheme B (1907308), Hong Kong Research Grant Council-Early Career Scheme (24115815) and -Collaborative Research Fund (C4017-14G). William Wu and Alfred Cheng are supported by funding from the Young Researcher Award, The Chinese University of Hong Kong.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Bo Zhai (Department of Hepatobiliary Surgery, The Fourth Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 2012;10:717-28. [Crossref] [PubMed]
- Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol 2012;39:461-72. [Crossref] [PubMed]
- Oikawa T. Cancer Stem cells and their cellular origins in primary liver and biliary tract cancers. Hepatology 2016;64:645-51. [Crossref] [PubMed]
- Chai S, Ng KY, Tong M, et al. Octamer 4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in liver cancer stem cells. Hepatology 2016;64:2062-76. [Crossref] [PubMed]
- Tian Y, Mok MT, Yang P, et al. Epigenetic Activation of Wnt/β-Catenin Signaling in NAFLD-Associated Hepatocarcinogenesis. Cancers (Basel) 2016;8:E76 [Crossref] [PubMed]
- Cheng AS, Lau SS, Chen Y, et al. EZH2-mediated concordant repression of Wnt antagonists promotes β-catenin-dependent hepatocarcinogenesis. Cancer Res 2011;71:4028-39. [Crossref] [PubMed]
- Tian Y, Wong VW, Wong GL, et al. Histone Deacetylase HDAC8 Promotes Insulin Resistance and β-Catenin Activation in NAFLD-Associated Hepatocellular Carcinoma. Cancer Res 2015;75:4803-16. [Crossref] [PubMed]
- Feng H, Cheng AS, Tsang DP, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives β-catenin/T cell factor-dependent hepatocarcinogenesis. J Clin Invest 2011;121:3159-75. [Crossref] [PubMed]
- Yu Z, Gao YQ, Feng H, et al. Cell cycle-related kinase mediates viral-host signalling to promote hepatitis B virus-associated hepatocarcinogenesis. Gut 2014;63:1793-804. [Crossref] [PubMed]
- Feng H, Yu Z, Tian Y, et al. A CCRK-EZH2 epigenetic circuitry drives hepatocarcinogenesis and associates with tumor recurrence and poor survival of patients. J Hepatol 2015;62:1100-11. [Crossref] [PubMed]
- Simandi Z, Horvath A, Wright LC, et al. OCT4 Acts as an Integrator of Pluripotency and Signal-Induced Differentiation. Mol Cell 2016;63:647-61. [Crossref] [PubMed]
- Zhu P, Wang Y, He L, et al. ZIC2-dependent OCT4 activation drives self-renewal of human liver cancer stem cells. J Clin Invest 2015;125:3795-808. [Crossref] [PubMed]
- Raza U, Zhang JD, Sahin O. MicroRNAs: master regulators of drug resistance, stemness, and metastasis. J Mol Med (Berl) 2014;92:321-36. [Crossref] [PubMed]
- Ji J, Yamashita T, Budhu A, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 2009;50:472-80. [Crossref] [PubMed]
- Ma S, Tang KH, Chan YP, et al. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 2010;7:694-707. [Crossref] [PubMed]
- Zhou L, Yang ZX, Song WJ, et al. MicroRNA-21 regulates the migration and invasion of a stem-like population in hepatocellular carcinoma. Int J Oncol 2013;43:661-9. [PubMed]
- Qian NS, Liu WH, Lv WP, et al. Upregulated microRNA-92b regulates the differentiation and proliferation of EpCAM-positive fetal liver cells by targeting C/EBPß. PLoS One 2013;8:e68004 [Crossref] [PubMed]
- Song K, Kwon H, Han C, et al. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: regulation by MIR-122. Oncotarget 2015;6:40822-35. [PubMed]
- Chai S, Tong M, Ng KY, et al. Regulatory role of miR-142-3p on the functional hepatic cancer stem cell marker CD133. Oncotarget 2014;5:5725-35. [Crossref] [PubMed]
- Yan H, Dong X, Zhong X, et al. Inhibitions of epithelial to mesenchymal transition and cancer stem cells-like properties are involved in miR-148a-mediated anti-metastasis of hepatocellular carcinoma. Mol Carcinog 2014;53:960-9. [PubMed]
- Li L, Liu Y, Guo Y, et al. Regulatory MiR-148a-ACVR1/BMP circuit defines a cancer stem cell-like aggressive subtype of hepatocellular carcinoma. Hepatology 2015;61:574-84. [Crossref] [PubMed]
- Zhang J, Luo N, Luo Y, et al. microRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c-Myb. Int J Oncol 2012;40:747-56. [PubMed]
- Ji J, Zheng X, Forgues M, et al. Identification of microRNAs specific for epithelial cell adhesion molecule-positive tumor cells in hepatocellular carcinoma. Hepatology 2015;62:829-40. [Crossref] [PubMed]
- Yan-Chun L, Hong-Mei Y, Zhi-Hong C, et al. MicroRNA-192-5p Promote the Proliferation and Metastasis of Hepatocellular Carcinoma Cell by Targeting SEMA3A. Appl Immunohistochem Mol Morphol 2015; [Epub ahead of print]. [Crossref] [PubMed]
- Liu J, Ruan B, You N, et al. Downregulation of miR-200a induces EMT phenotypes and CSC-like signatures through targeting the β-catenin pathway in hepatic oval cells. PLoS One 2013;8:e79409 [Crossref] [PubMed]
- Wang J, Yang X, Ruan B, et al. Overexpression of miR-200a suppresses epithelial-mesenchymal transition of liver cancer stem cells. Tumour Biol 2015;36:2447-56. [Crossref] [PubMed]
- Xia H, Ooi LL, Hui KM. MiR-214 targets β-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One 2012;7:e44206 [Crossref] [PubMed]
- Wu K, Ding J, Chen C, et al. Hepatic transforming growth factor beta gives rise to tumor-initiating cells and promotes liver cancer development. Hepatology 2012;56:2255-67. [Crossref] [PubMed]
- Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology 2013;58:629-41. [Crossref] [PubMed]
- Li L, Tang J, Zhang B, et al. Epigenetic modification of MiR-429 promotes liver tumour-initiating cell properties by targeting Rb binding protein 4. Gut 2015;64:156-67. [Crossref] [PubMed]
- Zheng Z, Liu J, Yang Z, et al. MicroRNA-452 promotes stem-like cells of hepatocellular carcinoma by inhibiting Sox7 involving Wnt/β-catenin signaling pathway. Oncotarget 2016;7:28000-12. [PubMed]
- Zhang X, Jiang P, Shuai L, et al. miR-589-5p inhibits MAP3K8 and suppresses CD90+ cancer stem cells in hepatocellular carcinoma. J Exp Clin Cancer Res 2016;35:176. [Crossref] [PubMed]
- Smallwood SA, Lee HJ, Angermueller C, et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods 2014;11:817-20. [Crossref] [PubMed]
- Klein AM, Mazutis L, Akartuna I, et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 2015;161:1187-201. [Crossref] [PubMed]